0081
Fat mitigation strategies to improve image quality of radial 4D flow MRI in obese subjects1Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology and Nuclear Medicine, Universität zu Lübeck, Lübeck, Germany, 3Department of Electrical and Computer Engineering, University of Wisconsin-Madison, Madison, WI, United States, 4Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 5Department of Mechanical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 7Department of Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
4D flow MRI of the abdomen is challenging in obese patients due to confounding streak artifacts from fat and large imaging volume. This ongoing study aims to test different fat mitigation strategies to improve image quality. We acquired three 10-min contrast-enhanced, radial 4D flow sequences: 1) with clinically used standard parameters, 2) with inner volume excitation, and 3) with fat saturation. Preliminary results confirm best signal-to-noise ratio, longest streamlines and fewest streak-artifacts using inner volume excitation. Flow rate and repeatability did not differ between sequences.
Introduction
Abdominal 4D flow MRI offers comprehensive, non-invasive assessment of portal hemodynamics. It holds the potential to help diagnose liver disease and portal hypertension. Recent advances in accelerated acquisitions have enabled 4D flow imaging of the whole abdomen in reasonable scan times, as required to image the hepatic vasculature and potential portosystemic shunts.However, many patients with liver disease have an elevated body mass index (BMI), which poses challenges due to the high confounding signal from fat, larger required imaging volume, and chemical shift artifacts.1 These challenges are exacerbated when using accelerated imaging such as radial trajectories that lead to streak artifacts, and can also compromise background phase correction.2 The purpose of this study was to investigate the utilization of inner volume excitation3 and fat suppression in hepatic 4D flow MRI to improve image quality and flow quantification consistency in obese subjects.
Methods
Study cohort:In this IRB-approved, ongoing study, we enrolled 2 of the planned 20 obese volunteers with BMI>35. Subjects fast for 5 hours prior to the study and receive 3mg/kg intravenous ferumoxytol (Feraheme, AMAG Pharmaceuticals) given as slow infusion per ISMRM guidelines4 (off-label use). This ensures high vascular signal-to-noise ratio over the whole study due to its long intravascular half-life.5
Image Acquisition and Reconstruction:
The imaging protocol consists of three 4D flow acquisitions using a 5-point encoded 3D radially undersampled trajectory (PCVIPR6) covering the upper abdomen: 1) standard - without fat saturation (Std), 2) with inner volume excitation (IVE), 3) with fat saturation pulses played out every 15 TRs (FS). IVE is achieved with a 2D selective RF-pulse played out in the S/I- and L/R-directions. The 2ms 2D RF-pulse excites a 24cm field of view (FOV) in the L/R- and S/I-plane aimed to remove the signal from the arms and lower abdomen. Acquisition parameters common to all 3 acquisitions include: imaging volume=48x48x24cm; acquired resolution=1.25mm3 isotropic; flip=14˚; VENC=60cm/s. The number of spokes was adjusted for a scan time of 10min; retrospective respiratory gating; offline time-averaged reconstruction performed with PILS7; corrections for Maxwell terms and gradient non-linearity. TE/TR=2.4/6.5ms for Std and FS; TE/TR=2.2/7.6ms for IVE. Acquisitions are repeated after the subject sits up on the MRI table, resulting in 6 datasets per subject.
Data analysis:
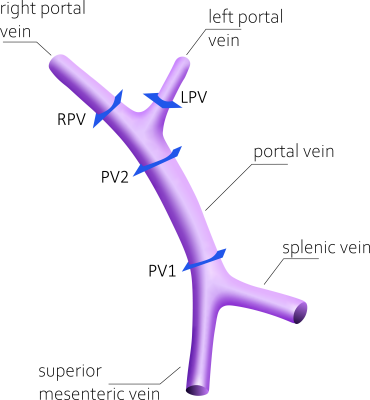
Third-order polynomial background phase correction8 was conducted using a custom MATLAB (Mathworks, Natick, MA) tool. Streamline visualization and flow measurements were obtained using EnSight (ANSYS, Canonsburg, PA). Volumetric flow rates were measured in the portal vasculature at 4 defined locations (Figure 1). For comparison of image quality, we used four scores: 1) consensus rating of image quality of time-averaged phase contrast angiograms by two expert readers, 2) streamline length, 3) conservation of mass analysis (COM-analysis), 4) repeatability of quantitative flow measurements. COM-analysis determined the relative error between volumetric flow rate (Q) in the distal portal vein compared to the sum of right and left portal vein: RE=1-[(QLPV+QRPV)/QPV2]. Volumetric flow rate between acquisitions were compared by Bland-Altman analysis and Pearson’s correlation.
Results
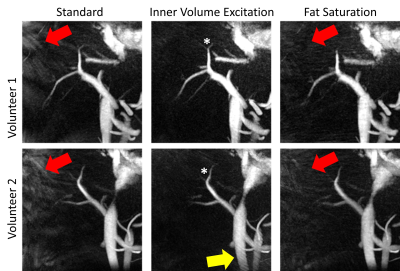
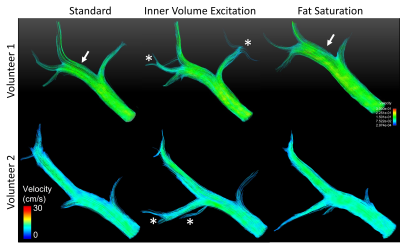
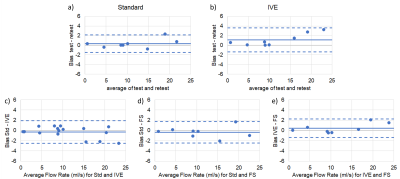
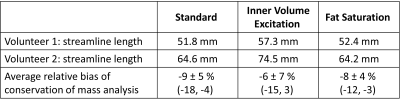
We successfully acquired data for the first two study participants (2 males, BMI=37.0 and 37.3; 50 and 54 years). Subjective image quality was rated best for IVE acquisition, followed by Std., Figure 2. Average streamline length was longest for IVE (Table 1). Std and FS had comparable length of streamlines (Figure 3). COM-analysis yielded comparable errors for all sequences (Table 1). Repeated measurements of all sequences were strongly correlated (r=0.99) with comparable bias and limits of agreement (Figure 4a-b). Results highly correlated between sequences (Std-IVE: r=0.99; Std-FS: r=0.99) with low bias and limits of agreement comparable to repeated measurements (Figure 4c-e).Discussion
We found improved image quality with inner volume excitation in radial 4D flow MRI of the hepatic vasculature compared to standard and fat saturated acquisitions. IVE notably reduced artifacts induced by the high confounding signal from fat because it limits the FOV to the actual region of interest. Further, it reduced the signal from the edge of the FOV where the gradient and magnetic field are non-linear.This is the first 4D flow MRI study applying inner volume excitation in the abdomen. Similar work in intracranial vessels3 proved to preserve velocity quantification with reduced FOV and therefore shortened acquisition time. Our work adds the improved image quality in obese subjects to the benefits of IVE.
For all sequences, we measured slightly higher mean errors of the conservation of mass analysis (6-9%) compared to previously reported values of 5-7%9-14. This was expected in the challenging cohort of severely obese subjects. Given the improved image quality of IVE, we assume that we could find improved flow quantification results of IVE compared to FS and Std after inclusion of more subjects.
The main limitation of IVE is a longer excitation time and a lower time bandwidth product RF-pulse. This results in a longer TR and hence fewer projections acquired given a fixed scan time, although this is compensated for by the smaller effective FOV. It further may increase sensitivity to poor shimming which is yet to be evaluated in a larger cohort.
Conclusion
Preliminary results confirm improved image quality with inner volume excitation for radial 4D flow MRI of the hepatic vasculature compared to standard and fat saturated acquisitions.Acknowledgements
The authors wish to acknowledge the NIH (R01 DK125783) for supporting this study, as well as GE Healthcare who provides research support to the University of Wisconsin. Dr. Oechtering receives funding from the German Research Foundation (OE 746/1-1). Dr. Reeder is a Romnes Faculty Fellow and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.
References
1. Middione MJ, Ennis DB. Chemical shift-induced phase errors in phase-contrast MRI. Magnetic Resonance in Medicine 2013;69:391-401.
2. Johnson KM, Wieben O, Samsonov AA. Phase-contrast velocimetry with simultaneous fat/water separation. Magnetic Resonance in Medicine 2010;63:1564-74.
3. Wink C, Ferrazzi G, Bassenge JP, et al. 4D flow imaging with 2D-selective excitation. Magn Reson Med 2019;82:886-900.
4. Vasanawala SS, Nguyen KL, Hope MD, et al. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med 2016;75:2107-11.
5. Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging 2015;41:884-98.
6. Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med 2008;60:1329-36.
7. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic Resonance in Medicine 2002;47:1202-10.
8. Walker PG, Cranney GB, Scheidegger MB, Waseleski G, Pohost GM, Yoganathan AP. Semiautomated method for noise reduction and background phase error correction in MR phase velocity data. J Magn Reson Imaging 1993;3:521-30.
9. Roldan-Alzate A, Frydrychowicz A, Niespodzany E, et al. In vivo validation of 4D flow MRI for assessing the hemodynamics of portal hypertension. J Magn Reson Imaging 2013;37:1100-8.
10. Motosugi U, Roldan-Alzate A, Bannas P, et al. Four-dimensional Flow MRI as a Marker for Risk Stratification of Gastroesophageal Varices in Patients with Liver Cirrhosis. Radiology 2019;290:101-7.
11. Roldan-Alzate A, Frydrychowicz A, Said A, et al. Impaired regulation of portal venous flow in response to a meal challenge as quantified by 4D flow MRI. J Magn Reson Imaging 2015;42:1009-17.
12. Roberts GS, Francois CJ, Starekova J, Roldan-Alzate A, Wieben O. Non-invasive assessment of mesenteric hemodynamics in patients with suspected chronic mesenteric ischemia using 4D flow MRI. Abdom Radiol (NY) 2021.
13. Brunsing RL, Brown D, Almahoud H, et al. Quantification of the Hemodynamic Changes of Cirrhosis with Free-Breathing Self-Navigated MRI. J Magn Reson Imaging 2021.
14. Stankovic Z, Jung B, Collins J, et al. Reproducibility study of four-dimensional flow MRI of arterial and portal venous liver hemodynamics: influence of spatio-temporal resolution. Magn Reson Med 2014;72:477-84.
Figures