0079
Automated compensation of respiratory motion and bulk patient movement in free-running whole-heart 4D MRI1Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 3Woman-Mother-Child, Pediatric Cardiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 4Cardiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 5Advanced Clinical Imaging Technology (ACIT), Siemens Healthcare AG, Lausanne, Switzerland
Synopsis
A novel methodology for whole-heart 4D MRI with retrospective compensation of both respiratory motion and bulk patient movement is developed and its initial feasibility demonstrated. This approach enables imaging with high isotropic resolution and allows for retrospective evaluation of the dynamic cardiac anatomy creating a new way to significantly improve image quality in un-cooperative patients and potentially decreasing the need for sedation.
Background
Conventional 3D whole-heart MRI requires ECG gating and respiratory navigation to obtain high resolution images. However, this leads to unpredictable acquisition times and adds complexity to scan planning. Recently, the free-running framework (FRF) was proposed to simplify cardiac MRI workflow by acquiring 3D whole-heart data continuously for a fixed scan time and then retrospectively reconstructing fully self-gated cardiac and respiratory motion-resolved (5D) images (1). Despite the advantages of this approach, FRF acquisition times are typically several minutes long and therefore bulk patient movement may significantly deteriorate image quality leading to the possible need for patient sedation, especially in children. In this work we present a novel methodology that identifies and compensates for both respiratory motion and bulk patient movement in FRF data sets to reconstruct high-quality dynamic 4D images. We validate this approach using a numerical simulation and demonstrate its ability to improve image quality relative to uncorrected reconstructions. We evaluate its feasibility in a cohort of patients exhibiting unpredictable bulk movement during the scan.Methods
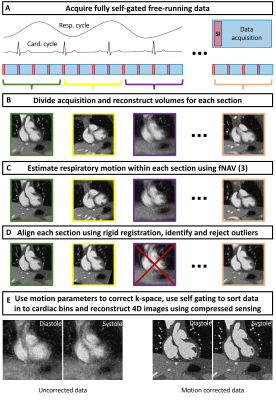
A schematic of our proposed methodology for dynamic whole-heart images in the presence of motion is shown in Fig. 1 and includes translational correction of respiratory motion using focused navigation (fNAV) (2), rigid correction of bulk movement, and outlier rejection (3). To validate our approach, a comprehensive numerical simulation of whole-heart MRI data was implemented as previously described (2,4), and included realistic programable cardiac and respiratory motion, as well as bulk movement. Simulated data acquisition parameters were set to match the in vivo data described below. A total of 100 simulation trials each with randomized levels of bulk motion were performed. The error between fNAV estimations of respiratory motion amplitude in each spatial direction relative to ground truth was quantified, as was the error in the estimated translational and rotational components of bulk movement relative to ground truth. Image sharpness was also measured and compared between uncorrected, corrected, and motion-free images (5). To demonstrate the feasibility of our approach in vivo, ten patients with congenital heart disease, (age: 6-23 years, 4 males), with a clinical indication for cardiac MRI, were included in this IRB approved study. These subjects are part of a larger research study and were included in this work based on visually identified bulk motion using the first steps of the framework described in Fig. 1. Examinations were performed without sedation, during free breathing, on a 1.5T clinical MRI system (MAGNETOM Sola, Siemens Healthcare, Erlangen, Germany) after administration of 2 mg/kg of ferumoxytol. A slab-selective spoiled gradient echo prototype free-running 3D radial sequence was used and resulted in uninterrupted acquisitions of six minutes duration (1,6). Main sequence parameters were RF excitation angle: 15°, resolution: (1.15 mm)3, FOV (220 mm)3, TE/TR: 1.53/2.84 ms, readout bandwidth: 1002 Hz/pixel. All datasets were reconstructed using the previously described approach for cardiac and respiratory resolved 5D imaging (1) and with the proposed method for motion compensated 4D imaging described in Fig. 1. Subsequent comparisons were made between the end-expiratory phase of the 5D images and the proposed 4D images reconstructed from the same data sets. Comparison of image quality was performed using an artificial-intelligence based algorithm trained to grade 3D radial images of the heart (7) and statistical significance was measured using a paired t-test.Results
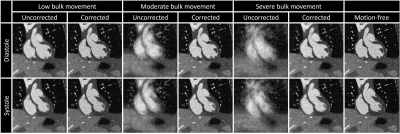
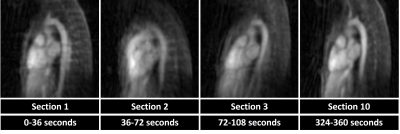
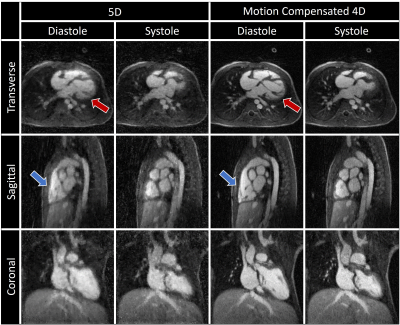
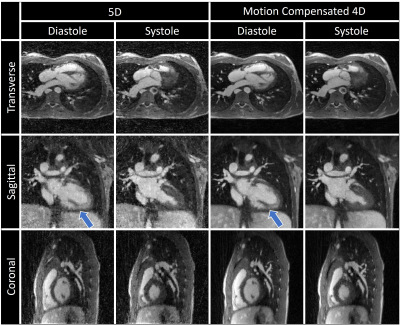
Fig. 2 illustrates the effect different levels of simulated motion has on image reconstructions. Overall, image quality is well preserved using motion correction which is reflected by the quantitative measurements from the numerical framework as follows. The error in the fNAV estimations of respiratory motion amplitude were 0.31±0.14 mm, 0.18±0.14 mm, and 0.18±0.10 mm for the x, y, and z directions respectively. The error in the estimations of bulk movement were 0.12±0.07 mm, 0.09±0.03 mm, and 0.17±0.10 mm for the translational components along each direction and 0.04±0.02 °, 0.07±0.03 °, and 0.04±0.01 ° for rotational components about each axis. The image sharpness measurements were 16.8±1.4 % for uncorrected, 37.1±1.2 % for corrected, and 41.0±0.7 % for motion-free. Fig. 3 demonstrates the level of bulk patient movement that can occur during the scan and subsequently identified using the proposed framework. Figs. 4 and 5 show 5D image reconstructions and the proposed motion compensated 4D reconstructions of representative patients who displayed severe (same patient shown in Fig. 3) and moderate bulk motion during the scan, respectively. Despite the varying levels of motion, the proposed algorithm provides excellent delineation of the dynamic cardiac anatomy and shows great improvement in image quality relative to the 5D images for both examples. When comparing all reconstructed data sets, the AI-based scoring of image quality yielded significantly (p<0.01) higher grades for the proposed 4D images (3.2±0.5) compared to 5D (2.5±1.0).Discussion and Conclusions
A novel methodology is proposed that produces high quality dynamic 3D images of the whole heart under free-breathing conditions and despite the presence of bulk patient movement. This approach has been validated in a comprehensive numerical simulation demonstrating high accuracy in the estimation and correction of the underlying motion. Overall, this work presents a new way to significantly improve image quality in un-cooperative patients potentially decreasing the need for sedation, especially in pediatric patients.Acknowledgements
The work was funded by the Swiss National Science Foundation (SNSF 320030B_201292)
References
1. Di Sopra L PD, Coppo S, Stuber M YJ. An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magn Reson Med. 2019;00:1:15.
2. Roy CW, Heerfordt J, Piccini D, et al. Motion compensated whole-heart coronary cardiovascular magnetic resonance angiography using focused navigation (fNAV). J. Cardiovasc. Magn. Reson. 2021;23:33 doi: 10.1186/s12968-021-00717-4.
3. Amerom JFP, Lloyd DF, Deprez M, et al. Fetal whole‐heart 4D imaging using motion‐corrected multi‐planar real‐time MRI. Magn. Reson. Med. 2019:1– 18 doi: 10.1002/mrm.27798.
4. Segars WP, Sturgeon G, Mendonca S, Grimes J, Tsui BMW. 4D XCAT phantom for multimodality imaging research. Med. Phys. 2010;37:4902 doi: 10.1118/1.3480985.
5. Etienne A, Botnar RM, van Muiswinkel AMC, Boesiger P, Manning WJ, Stuber M. Soap-Bubble visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn. Reson. Med. 2002;48:658–666 doi: 10.1002/mrm.10253.
6. Heerfordt J, Whitehead KK, Bastiaansen JAM, et al. Free-running SIMilarity-Based Angiography (SIMBA) for simplified anatomical MR imaging of the heart. 2020.
7. Piccini D, Demesmaeker R, Heerfordt J, et al. Deep Learning to Automate Reference-Free Image Quality Assessment of Whole-Heart MR Images. Radiol. Artif. Intell. 2020;2:e190123 doi: 10.1148/ryai.2020190123.
8. Schrauben EM, Lim JM, Goolaub DS, Marini D, Seed M, Macgowan CK. Motion robust respiratory‐resolved 3D radial flow MRI and its application in neonatal congenital heart disease. Magn. Reson. Med. 2020;83:535–548 doi: 10.1002/mrm.27945.
Figures