0078
Accelerated 2D PC-MRI using a Deep Learning-Based Reconstruction with Complex Difference Estimation: A Prospective Feasibility Study1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Bioengineering, Stanford University, Stanford, CA, United States, 3Cardiovascular Institute, Stanford University, Stanford, CA, United States
Synopsis
We have previously demonstrated a Deep Learning (DL) reconstruction of highly accelerated 2D PC-MRI datasets. We trained a DL reconstruction by retrospectively undersampling fully-sampled 2D PC-MRI datasets to enable up to 9x accelerated images with <5% error in accuracy and precision. Herein, we compare the accuracy and precision of 2D PC-MRI measurements for our prospectively deployed sequence and DL reconstruction. In this initial feasibility study, we show that our DL reconstruction provides <5% error in the accuracy of peak velocity and total flow relative to 2x parallel imaging and better accuracy and precision compared to 8x compressed sensing.
Introduction
2D Phase Contrast MRI (PC-MRI) allows for time-resolved measurements of blood velocity throughout the cardiovascular system. PC-MRI can be used clinically to derive important parameters of cardiovascular health and disease, including peak velocity (cm/s) and total flow (mL). However, 2D PC-MRI acquisitions require extended scan times that oftentimes necessitate patients to hold their breath for upwards of 20 seconds.Recently, Deep Learning (DL) based frameworks have been developed to reconstruct highly accelerated MRI acquisitions1-3. These DL-based methods can outperform conventional compressed sensing (CS)4-6 reconstruction methods. We have previously demonstrated a DL-based reconstruction that extended a DL-ESPIRiT framework7 developed for cardiac cine MRI to 2D PC-MRI datasets. In that work, we retrospectively undersampled the fully-sampled pediatric 2D PC-MRI datasets and trained a DL-based reconstruction network to enable up to 9x accelerated images with errors in accuracy and precision <5%, whereas CS methods produced errors >5% at 6x acceleration8,9.
The objective of this work was to perform an initial feasibility study of our prospectively deployed sequence and DL-based reconstruction framework by comparing the accuracy and precision in measurements of peak velocity and total flow in 2D PC-MRI using 2x in-plane parallel imaging, 8x CS, and the proposed 8x DL method.
Methods
Fully-sampled, raw k-space 2D PC-MRI pediatric datasets (n=194) were obtained with institutional IRB approval and patient consent. All imaging was performed using either a 1.5T (n=23) or 3.0T (n=171) system (GE Healthcare, Waukesha Wisconsin) and an ECG-gated PC-MRI sequence (Table 1). The fully-sampled datasets were randomly divided into training (80%), validation (5%), and testing (15%) cohorts with a 155/10/29 split. Variable-density undersampling masks with 6-11x acceleration across the ky-t plane were randomly generated and applied on-the-fly to each dataset during training. The network architecture is described in Fig. 1.2D PC-MRI datasets (n=3) were prospectively acquired using a custom ECG-gated spoiled gradient echo sequence with the following imaging parameters: field-of-view=360mm2, slice thickness=8mm, flip angle=25°, sup>. Measurements of peak velocity (mean value of the top 5% of all pixels contained in each vessel ROI) and total flow (mean vessel ROI velocity multiplied by the area of the vessel ROI integrated over the cardiac cycle) were compared.
Results
A qualitative comparison of the magnitude and phase images for 2x PI, 8x CS, and 8x DL demonstrates favorable agreement (Fig. 2). A quantitative comparison of peak velocity and total flow measurements across three repeated aortic valve scans in one volunteer and two repeated scans in both aortic and pulmonary valve scans of another volunteer show better agreement between 2x PI and 8x DL compared to 8x CS (Table 2). Specifically, 8x DL shows higher accuracy (lower mean percent difference relative to 2x PI) and higher precision (lower intra-scan variability) relative to 8x CS. Furthermore, accuracy was within 5% for 8x DL for both peak velocity and total flow, whereas accuracy was within 5% for 8x CS for total flow only. In the final volunteer, we compared the clinical utility of the 8x acceleration by exploring tradeoffs in scan time and spatiotemporal resolution. Mean velocity, peak velocity, and total flow were compared for: 1) reduced breath hold duration (Fig. 3A); 2) increased temporal resolution (Fig. 3B); and 3) increased spatial resolution (Fig. 3C).Discussion
The CS and DL images show a small artifact in the aorta of the magnitude images, visible as the aortic valve closes, and a slight exaggeration of pulsatility artifacts, visible both anteriorly and posteriorly to the chest wall in the phase images. However, this doesn’t seem to negatively impact the quantitative results. 8x CS shows underestimates of peak velocity, which aligns with the results of our retrospective analysis and other findings in the literature12. The additional acceleration afforded by 8x udersampling can be used to reduce breath hold durations and/or increase spatiotemporal resolution. While this initial feasibility study of our prospectively deployed 8x accelerated sequence and DL-based reconstruction framework shows promise, future work is needed to explore the clinical impact of the technique in clinical adult and pediatric patients.Conclusion
Initial feasibility results of prospectively undersampled 8x 2D PC-MRI datasets reconstructed with our proposed DL framework show improved accuracy and precision compared CS and <5% error in accuracy of peak velocity and total flow, relative to 2x PI.Acknowledgements
This project was supported, in part, by U01 EB029427 and support from GE Healthcare.References
1. Hammernik K, Klatzer T, Kobler E, Recht MP, Sodickson DK, Pock T, and Knoll F. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med. 2018;79(6):3055-3071.
2. Aggarwal HK, Mani MP, and Jacob M. MoDL: Model-based deep learning architecture for inverse problems. Magn Reson Med. 2018: 38(2):394-405.
3. Mardani M, Gong E, Cheng JY, Vasanawala SS, Zaharchuk G, Xing L, and Pauly JM. Deep generative adversarial neural networks for compressive sensing MRI. IEEE Trans Med Imag. 2018;38(1):167-179.
4. Lustig M, Donoho D and Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182-1195.
5. Giese D, Schaeffter T and Kozerke S. Highly undersampled phase‐contrast flow measurements using compartment‐based k–t principal component analysis. Magn Reson Med. 2013;69(2):434-443.
6. Sun A, Zhao B, Ma K, Zhou Z, He L, Li R, and Yuan C. Accelerated phase contrast flow imaging with direct complex difference reconstruction. Magn Reson Med. 2017;77(3):1036-1048.
7. Sandino CM, Lai P, Vasanawala SS, Cheng JY. Accelerating cardiac cine MRI using a deep learning‐based ESPIRiT reconstruction. Magn Reson Med. 2021;85(1):152-67.
8. Oscanoa JA, Middione MJ, Sandino CM, Vasanawala SS, Ennis DB. Accelerated 2D Phase Contrast MRI using deep learning-based reconstruction and direct complex difference estimation. SCMR 24th Annual Meeting, 2021.
9. Oscanoa JA, Middione MJ, Sandino CM, Vasanawala SS, Ennis DB. Deep Learning Based ESPIRiT Reconstruction for Highly Accelerated 2D Phase Contrast MRI. ISMRM 28th Annual Meeting, 2021.
10. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, and Lustig M. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001.
11. Lankhaar JW, Hofman MB, Marcus JT, Zwanenburg JJ, Faes TJ, Vonk‐Noordegraaf A. Correction of phase offset errors in main pulmonary artery flow quantification. J Magn Reson Imag. 2005;22(1):73-9.
12. Ma LE, Markl M, Chow K, Huh H, Forman C, Vali A, Greiser A, Carr J, Schnell S, Barker AJ, Jin N. Aortic 4D flow MRI in 2 minutes using compressed sensing, respiratory controlled adaptive k‐space reordering, and inline reconstruction. Magn Reson Med. 2019;81(6):3675-90.
Figures
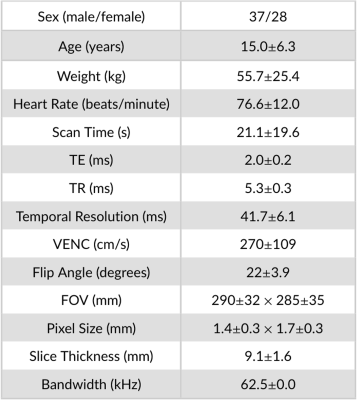
Table 1. Patient demographics and 2D PC-MRI imaging parameters used to train, evaluate, and test the retrospectively undersampled 2D PC-MRI DL-based network (n=194).
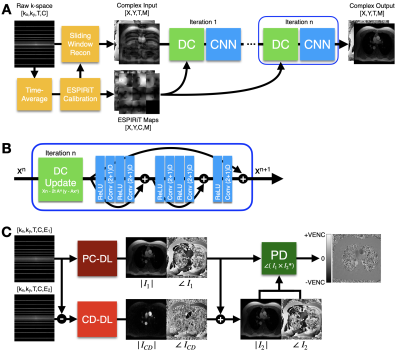
Figure 1. (A) The DL reconstruction pipeline uses an unrolled network architecture that iteratively alternates between a Data Consistency (DC) update and a CNN-based denoising step. (B) The CNN was comprised of a Rectified Linear Unit (ReLU) pre‐activation layer and a 2D spatial and 1D temporal (2+1)D convolutional layer, as described in the original DL-ESPIRiT methodology7. (C) The first velocity encoding and a complex difference (CD) image are reconstructed using PC-DL and CD-DL, respectively. The final velocity image is generated phase difference processing (PD).
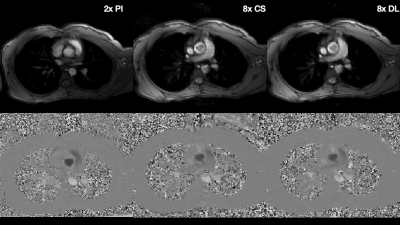
Figure 2. Qualitative results from a single volunteer showing the magnitude (top) and phase (bottom) for conventional 2x PI as well as 8x CS and the proposed DL method. Overall, image quality is preserved for the CS and DL reconstruction methods, with a small artifact in the aorta of the magnitude images, visible as the aortic valve closes, and a slight exaggeration of pulsatility artifacts, visible both anteriorly and posteriorly to the chest wall in the phase images.
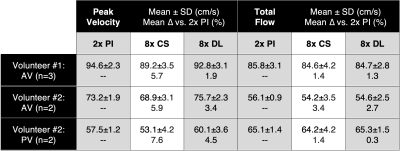
Table 2. Quantitative comparison of peak velocity and total flow. Comparisons were made for 2x PI scans as well as 8x accelerated scans reconstructed with CS and the proposed DL method. 8x DL shows higher accuracy (lower mean % difference) and higher precision (smaller standard deviation) relative to 8x CS. Furthermore, accuracy is within 5% for 8x DL for both peak velocity and total flow, whereas accuracy is within 5% for 8x CS for total flow only.
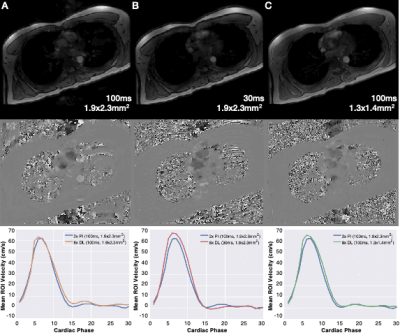
Figure 3. Qualitative results from a single volunteer showing the magnitude (top) and phase (middle) images for 8x DL reconstructed images for (A) reduced scan time (20 seconds vs. 5 seconds), (B) increased temporal resolution (100ms vs. 30ms) and (C) increased spatial resolution (1.9x2.3mm2 vs. 1.3x1.4mm2).