0076
Exercise-induced hyperemia ASL and CrCEST at 3T demonstrate individual calf muscle improvement post-revascularization in patients with PAD1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Cardiac Imaging, University of Virginia, Charlottesville, VA, United States, 3Cardiology, University of Virginia, Charlottesville, VA, United States, 4Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States
Synopsis
Peripheral arterial disease is a prevalent atherosclerotic disease characterized by atherosclerotic lesions in the limbs. Patients with PAD have been shown to have a delayed phosphocreatine recovery due to chronic ischemia. Patients with PAD may be candidates for revascularization, but outcomes are variable. CrCEST allows for creatine concentrations to be monitored at high spatial resolution, while ASL quantifies perfusion into tissue. We use this combination to revascularization in patients undergoing both endovascular and surgical procedures. Preliminary results link improvement in CrCEST decay curves and improved functional walk scores, while increased perfusion seen on ASL does not necessarily correlate with improvement.
Introduction
Peripheral arterial disease (PAD) affects around 10% of individuals over age 40 in the US and is characterized by atherosclerotic lesions in the arteries supplying one or more limbs, leading to decreased perfusion in downstream tissue.[1] Patients with PAD have been shown to have a delayed phosphocreatine recovery, which suggests impaired skeletal muscle metabolism due to chronic ischemia. Creatine chemical exchange saturation transfer (CrCEST) is an MRI technique that utilizes radiofrequency pulses to magnetically label protons that are exchanged between creatine and water, allowing for creatine concentrations to be monitored at high spatial resolution. During recovery from exercise, the creatine concentration decays as it is converted to phosphocreatine in the mitochondria of the skeletal muscle. Arterial spin labeling (ASL) uses tagged inverted arterial blood as endogenous contrast to visualize and quantify perfusion into tissue. Combining ASL and CrCEST in the same protocol allows for spatial visualization of metabolism and perfusion. Patients with Intermittent claudication (IC) have PAD symptoms that resolve during rest, while critical limb ischemia (CLI) is a subset of severe PAD that presents with pain at rest, ulceration, or gangrene. Patients with either type of PAD may be candidates for a revascularization procedure. The goal of this study is to use this combination to assess changes after revascularization in patients undergoing both endovascular and surgical procedures and evaluate the recovery of both perfusion and metabolism in their muscle tissue.Methods
Subjects with known PAD (defined as claudication with confirmed ankle-brachial index<0.9) scheduled for revascularization were enrolled. All underwent MRI on a 3T PRISMA Siemens scanner using a transmit-receive knee coil. All subjects underwent baseline imaging in which water saturation with shift reference (WASSR) and B1 maps were collected for B0 and B1 correction.[2] Six images were then acquired over 24 second intervals with saturation frequency offsets of ±1.3, ±1.8, and ±2.3 ppm. The CEST effect reduces the signal at +1.8 ppm compared to -1.8 ppm, referred to as CrCESTasym. Subjects performed plantarflexion exercise on an MR-compatible ergometer (Ergospect, NL) until calf exhaustion at which point post-exercise images were obtained. CrCESTasym maps were obtained with a region of interest (ROI) drawn around the anterior tibialis, posterior tibialis, and gastrocnemius muscles. Creatine decay times were obtained by fitting an exponential curve to the CrCESTasym values. For the ASL imaging, control-tagged image pairs were acquired repetitively using a PASL pulse sequence .The tag was applied 35 mm proximal to the first imaging slice for 1200 ms, followed by a 1500 ms post-label delay.[3] Motion correction was performed between temporal frames, and relative blood flow maps were calculated using a simplified single compartment ASL model.[4] An ROI was drawn around the muscle group demonstrating the highest perfusion and compared to the corresponding ROI on the CrCESTasym map.Results
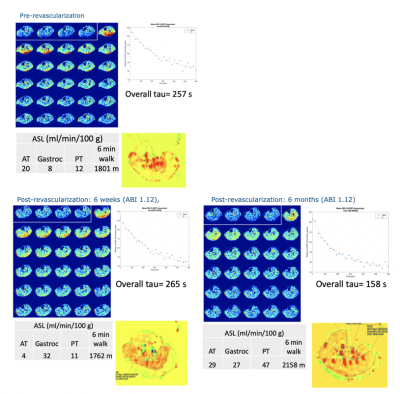
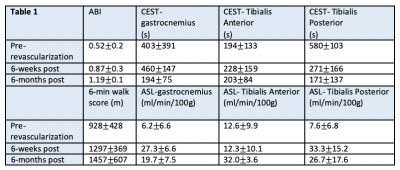
Seven PAD patients were imaged before and after revascularization. Due to COVID-19 pandemic precautions, follow-up imaging has been problematic for some patients. Data from a patient who has returned at all time points is shown in Figure 1. This patient underwent a posterior approach to left popliteal-popliteal bypass using reversed great saphenous vein, after which the ABI improved from 0.71 to 1.12. The ASL perfusion shows an increase in flow to the gastrocnemius, with a restriction of flow to the rest of the muscle groups at 6 weeks, that becomes a uniform increase throughout the calf at 6 months. The tau time-constant of the CrCEST decay initially elongates at 6-weeks post revascularization, when the 6-minute walk score also worsens. At 6 months the decay shortens and the walk score improves. Results of all patients returning at 6 weeks (n=5) or 6 months (n=3) are shown in Table 1. There appears to be an initial worsening of CrCEST decays at 6 weeks, followed by an improvement at 6 months. ASL perfusion increases immediately post-revascularization along with ABI, but there is more uniform flow at 6 months compared to 6 weeks.Discussion
This combination of ASL and CrCEST provides a novel methodology to compare metabolism and perfusion on a muscle-group specific basis. Preliminary data suggest it takes 6 months for full recovery of calf muscle perfusion and energetics after revascularization. Further follow-up will enable the comparison of the utility of CrCEST and ASL in correlation to functional outcomes. This study will be the first to investigate the relationship between metabolism and perfusion on a muscle-by-muscle basis. It should lead to a better understanding of PAD and could lead to improved treatment planning for PAD patients.Acknowledgements
No acknowledgement found.References
(1) Virani SS, Alonso A, Aparicio HJ, et al, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021 Feb 23;143(8):e254-e743. doi: 10.1161/CIR.0000000000000950. Epub 2021 Jan 27. PMID: 33501848.
(2) Kogan F, Haris M, Debrosse C, et al. In vivo chemical exchange saturation transfer imaging of creatine (CrCEST) in skeletal muscle at 3T. J Magn Reson Imaging. 2014;40(3):596-602. doi:10.1002/jmri.24412
(3) Pollak AW, Meyer CH, Epstein FH et al. Arterial Spin Labeling MR Imaging Reproducibly Measures Peak-Exercise Calf Muscle Perfusion: A Study in Patients With Peripheral Arterial Disease and Healthy Volunteers. JACC: Cardiovascular Imaging 2012 December;5(12):1224-30.
(4) Grozinger G, Pohmann R, Schick F et al. Perfusion measurements of the calf in patients with peripheral arterial occlusive disease before and after percutaneous transluminal angioplasty using Mr arterial spin labeling. J Magn Reson Imaging2014 October 1;40(4):980-7.
Figures