0075
Velocity-selective arterial spin labeling perfusion in the evaluation of treated high grade gliomas at 1.5 Tesla1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Institute of Neuroradiology, University Hospital LMU Munich, Munich, Germany, 3F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 4Department of Oncology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Perfusion measures were compared between VSASL and DSC methods at 1.5T in 28 patients with treated high-grade glioma assigned into 2 groups: “tumor” (with detectable enhancing tumor, n=9) and “non-tumor” (without detectable tumor, n=19). All measures (rCBF and tSNR from VSASL, rCBV and rCBF from DSC) showed significant difference between “tumor” and “non-tumor” groups allowing reliable discrimination. In general, there was moderate to excellent agreement and correlation between these measures derived from VSASL vs. DSC. Hence, VSASL has potential to serve as a viable non-invasive alternative to DSC perfusion in the clinical disease surveillance without the need for exogenous contrast.
Introduction
MR perfusion imaging provides important hemodynamic information and has become an integral component of the clinical evaluation of primary brain gliomas particularly following treatment.1-5 Velocity selective arterial spin labeling (VSASL) is a non-invasive technique of perfusion mapping without the use of exogenous contrast agent and may serve as a viable alternative to the more widely implemented dynamic susceptibility contrast (DSC) perfusion methods.6-8 The aim of this study was to assess the utility of VSASL compared to DSC in the routine clinical surveillance MR exams of patients with high grade gliomas at 1.5 Tesla.Methods
Twenty-eight patients with treated brain glioma (16 WHO Grade IV, 12 WHO Grade III) presented for routine follow-up clinical MRI consented to a 10-minute additional scan of VSASL tagged onto the contrast exam including DSC perfusion at 1.5 Tesla. “Tumor” (detectable tumor) and “non-tumor” (no detectable tumor) groups were classified using standard RANO criteria based on the index exam and subsequent follow-up MRIs. ASL data was post-processed using MATLAB to generate relative cerebral blood flow (rCBF) and temporal signal-to-noise ratio (tSNR) maps. DSC perfusion raw data was processed using Olea Sphere to generate rCBF and relative cerebral blood volume (rCBV) with leakage correction. After co-registration with anatomic images, a region of interest (ROI) was drawn on tumor/lesion using ImageJ within areas of post contrast T1 and T2 FLAIR hyperintensity. The mean lesion rCBF/rCBV was calculated from automatically selecting 10 voxels with the highest perfusion values within the lesion ROI and normalized against the mean reference grey matter CBF in the contralateral unaffected brain. Perfusion values were compared between “tumor” and “non-tumor” cases using Mann-Whitney-test. Correlation of the perfusion measurements between VSASL rCBF and DSC rCBF were tested using Pearson’s correlation. Significance level was set at a=0.05.Results
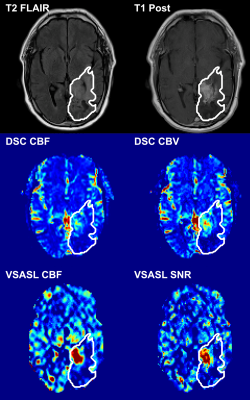
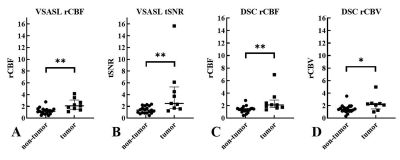
The 28 patients (12 female, mean age 55.2 years) were assigned into 2 groups: “tumor” (n=9, 100% IDH wildtype) and “non-tumor” (n=19, 13 with known IDH status: 69% IDH wildtype) based on the index MR exam according to RANO criteria. A representative patient with residual/recurrent tumor is shown in Figure 1, demonstrating elevated perfusion on both VSASL and DSC. Figure 2 shows that perfusion measures derived from both VSASL (rCBF and tSNR) and DSC (rCBF and rCBV) were able to discriminate “tumor” from “non-tumor” cases. Based on VSASL the normalized median ratio rCBF was 1.17 (IQR 0.81-1.52) in “non-tumor” cases, significantly lower than the 2.09 (1.52-3.09) found in “tumor” (p=0.0016, Mann-Whitney test); tSNR had a median value of 1.36 (0.87-2.10) in “non-tumor” compared to 2.50 (1.65-5.31) in “tumor” (p=0.0013). On DSC, the median normalized rCBF ratio was 1.39 (1.16-1.56) in “non-tumor” cases, significantly lower than the 2.11 (1.69-2.90) in “tumor” (p=0.0013); similarly, the median ratio rCBV measured 1.39 (1.12-1.68) in “non-tumor” compared to 2.19 (1.47-2.37) in “tumor” (p=0.022). Lin’s concordance (Figure 3) and Bland-Altman (Figure 4) plots show moderate to excellent concordance and agreement between VSASL and DSC methods.Discussion
This study shows the clinical applicability of VSASL in a cohort of patients with treated high-grade gliomas. There is a moderate to excellent correlation between VSASL and DSC perfusion parameters. The statistical analysis showed stronger moderate concordance between VSASL rCBF and DSC rCBV, the more common measurement in clinical evaluation of gliomas, than between VSASL rCBF and DSC rCBF, likely attributable to a more reliable estimate and lower noise level in DSC rCBV. While ASL in general has a low SNR, VSASL showed a superb SNR, whereby vascular tumors are particularly prominent in tSNR maps derived from VSASL. VSASL perfusion values have the ability to discriminate tumor progression from treatment effect comparable to DSC. Despite a longer acquisition time leading to more motion artifacts, which were mitigated by motion correction in post processing. VSASL is also more robust compared to spatially labelled ASL (e.g. pseudocontinuous ASL) technique at 1.5T; ASL degrades with field strength, but less severe for VSASL due to reduced sensitivity to arterial transit time. The limitations of this study are a relatively small cohort, unbalanced groups and the lack of definite histopathology.Conclusion
These first results indicate that VSASL is clinically feasible at 1.5 Tesla and has potential to offer a non-invasive alternative to DSC perfusion in monitoring disease in high grade gliomas following therapy. This is particularly valuable when Gadolinium contrast is contraindicated or undesirable. Further research is needed to validate this perfusion method in a larger cohort for its robustness in distinguishing active tumor from largely treatment effect, and to develop faster acquisition as well as streamlined post processing routines.Acknowledgements
No acknowledgement found.References
1. Boxerman JL, Shiroishi MS, Ellingson BM, Pope WB. Dynamic Susceptibility Contrast MR Imaging in Glioma: Review of Current Clinical Practice. Magn Reson Imaging Clin N Am 2016;24:649-670.
2. Law M, Young R, Babb J et al. Comparing Perfusion Metrics Obtained from a Single Compartment Versus Pharmacokinetic Modeling Methods Using Dynamic Susceptibility Contrast-Enhanced Perfusion MR Imaging with Glioma Grade. American Journal of Neuroradiology 2006;27:1975-1982.
3. Schmainda KM, Zhang Z, Prah M et al. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro Oncol 2015;17:1148-56.
4. van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol 2017;27:4129-4144.
5. Kruser TJ, Mehta MP, Robins HI. Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother 2013;13:389-403.
6. White CM, Pope WB, Zaw T et al. Regional and voxel-wise comparisons of blood flow measurements between dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) and arterial spin labeling (ASL) in brain tumors. J Neuroimaging 2014;24:23-30.
7. Jarnum H, Steffensen EG, Knutsson L et al. Perfusion MRI of brain tumours: a comparative study of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast imaging. Neuroradiology 2010;52:307-17.
8. Liu D, Xu F, Li W, van Zijl PC, Lin DD, Qin Q. Improved velocity-selective-inversion arterial spin labeling for cerebral blood flow mapping with 3D acquisition. Magn Reson Med 2020;84:2512-2522.
Figures
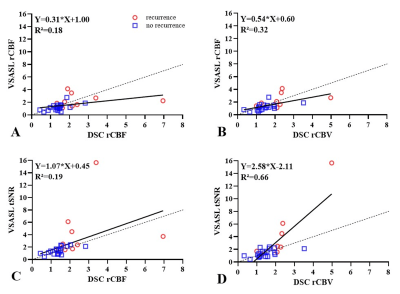
Lin’s concordance plots demonstrating moderate to excellent correlation between paired perfusion parameters, highest between DSC rCBV and VSASL tSNR.
A: DSC rCBF and VSASL rCBF, linear regression (Y=0.31*X+1.00; R²=0.18);
B: DSC rCBV and VSASL rCBF, linear regression (Y=0.54*X+0.60; R²=0.32);
C: DSC rCBF and VSASL tSNR, linear regression (Y=1.07*X+0.45; R²=0.19);
D: DSC rCBV and VSASL tSNR, linear regression (Y=2.58*X-2.11;R²=0.66)
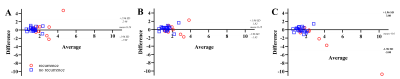
Bland-Altman-Plots showing good agreement between the perfusion parameters derived from VSASL vs. DSC
A: Difference: DSC rCBF – VSASL rCBF and average: (DSC rCBF + VSASL rCBF)/2;
B: Difference: DSC rCBV – VSASL rCBF and average: (DSC rCBV + VSASL rCBF)/2);
C: Difference: DSC rCBV – VSASL tSNR and average: (DSC rCBV + VSASL tSNR)/2)