0074
Elevated Foot Diffusion and Resting Perfusion in Patients with Diabetic Foot Ulcer: A Pilot and Feasibility Study1Emory University, Atlanta, GA, United States, 2Massachusetts General Hospital, Boston, MA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Here we apply intravoxel incoherent motion (IVIM) and fractional Fickian diffusion (FFD) models to multi-b-value diffusion-weighted-MRI to study tissue cellular and resting microvascular properties of the foot of patients with diabetic foot ulcer. We report preliminary results from an ongoing study comparing patients with type 2 diabetes and persistent plantar foot ulcers with healthy age- and BMI-matched individuals. Pseudo-diffusion and mean diffusion coefficients and microvascular volume fraction were elevated in patients, showing large effect sizes in subregions. This approach may prove useful for evaluating patients with ulcers to prognosticate tissue at risk of poor wound healing.
BACKGROUND:
Prediction of wound healing in diabetic foot ulcer (DFU) is clinically important to stratify the risk of amputation and target limb salvage interventions. Current guidelines recommend non-invasive vascular status assessments, such as the ankle-brachial index, for all patients with diabetic foot ulcers (DFU) [1, 2]. However, these tests have poor predictive value for wound healing [3] and have been shown to be poorly correlated with tissue perfusion in DFU as measured by more advanced MRI approaches [4]. Diffusion-weighted MRI provides indices of resting tissue diffusion and perfusion that have demonstrated sensitivity to tissue changes in skeletal muscle of the lower extremities [5]. In this pilot study, we examine the sensitivity of diffusion-weighted-MRI measurements to changes in the diabetic foot with persistent plantar ulcers. This ongoing study focuses on developing MR imaging metrics that can prognosticate impaired wound healing in the diabetic foot with ulcer.METHODS:
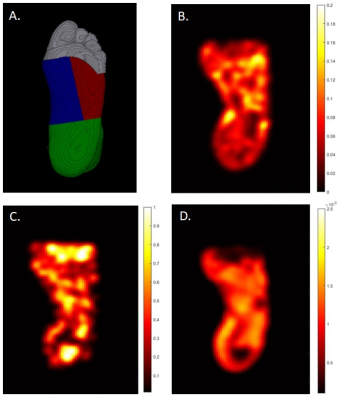
A total of 10 participants, N=5 diabetic foot ulcer (DFU) patients with persistent wounds and N=5 matched healthy controls (HC), are included in this preliminary analysis. Subjects were recruited under an approved institutional IRB. Selection criteria for DFU patients consisted of diagnosis of type II diabetes with presence of an unhealed plantar foot ulcer for at least one month. Patients were excluded if there was evidence of active osteomyelitis, indication of macrovascular disease or chronic kidney disease greater than stage 3. All imaging was performed in the early morning after an overnight fast. Imaging experiments were conducted on a 3 Tesla MRI (MAGNETOM Prisma Fit, Siemens Healthcare, Erlangen, Germany) with a 20-channel head coil for signal reception. Subjects were positioned supine feet-first with ipsilateral foot secured inside the coil and contralateral foot secured outside the coil. T1-weighted images of the entire foot were acquired as an anatomical reference. Diffusion-weighted-MRI was acquired using a prototype diffusion sequence with the following parameters: TR/TE=7800/65ms; FOV=320mm; 46 slices, thickness=2.2mm; in-plane resolution=2.5×2.5mm2; 4 averages; spectral adiabatic inversion recovery fat suppression; and 3 orthogonal diffusion directions, with b=0, 5, 10, 15, 20, 40, 80,160, 320, 800s/mm2. In-line distortion correction was performed on the scanner and trace-weighted images were used for further post-processing. Anatomic images were co-registered with trace-weighted images using MRIcroGL and used to define regions of interest consisting of the toes, medial plantar forefoot, and lateral plantar forefoot (Figure 1)[6]. Intravoxel incoherent motion (IVIM) analysis was performed using a two-step fitting approach [7] whereby: (1) mean diffusivity (D) was fit to a single exponential decay using b-values greater than 150s/mm2; and (2) perfusion fraction (fp) and pseudo-diffusion coefficient (D*) were fit with a bi-exponential model with D fixed to the value in step one. Microvascular volume fraction was derived from fits to the fractional Fickian diffusion (FFD) model [8] correcting for relaxation differences between tissue and blood [9]. Briefly, the stretched exponential function was fit to diffusion decay data yielding estimated parameters that were used to compute the cumulative distribution function of the diffusion propagator and microvascular volume fraction (MVF) was computed based on previously defined velocity cut-offs [10].RESULTS:
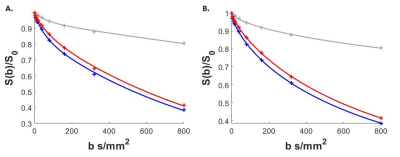
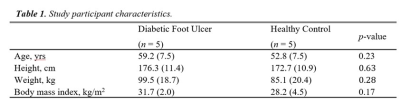
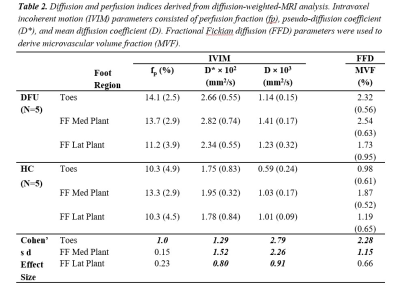
Table 1 shows participant characteristics with DFU and HC groups matched by age and BMI. Figure 1. (B-D) illustrates typical IVIM parameter maps from a single slice in the plantar region of the foot. Figure 2 shows the fit quality of both IVIM and FFD models to representative data from each foot region. Fit residuals were within 1.5% of the total signal suggesting good data and fit quality. DFU patients tended to show elevated tissue diffusion and perfusion indices compared with healthy controls. Table 2 shows that the largest mean differences between groups occurs in the toes with effect sizes fp=1, D*=1.29, D=2.79, and MVF=2.28. The medial plantar forefoot also showed large effect sizes with D*=1.52, D=2.26, and MVF=1.15.DISCUSSION:
Our observations using the IVIM and FFD models suggest increased fluid volume both within the tissue space (i.e. intra- and extra-cellular) and microvascular space in DFU patients compared with HC. This is consistent with gross clinical observation of hyper-perfusion in the foot. Our measures demonstrate regional variation in model indices with the largest deviations in the toes and medial plantar regions, consistent with most common presentations of foot ulcers. Effect sizes reported in this work support further investigation into the use of diffusion-weighted MRI to forecast likelihood of wound healing in patients with diabetic foot ulcers. Ongoing work will compare diffusion and perfusion metrics to longitudinal measures of wound healing and blood markers of inflammation.Acknowledgements
Financial support for this work was provided by the NIDDK Diabetic Complications Consortium (RRID:SCR_001415, www.diacomp.org), grants DK076169 and DK115255.References
1. Hingorani, A., et al., The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg, 2016. 63(2 Suppl): p. 3S-21S.
2. Lipsky, B.A., et al., 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis, 2012. 54(12): p. e132-73.
3. Brownrigg, J.R., et al., Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev, 2016. 32 Suppl 1: p. 128-35.
4. Edalati, M., et al., Intravenous contrast-free standardized exercise perfusion imaging in diabetic feet with ulcers. J Magn Reson Imaging, 2018.
5. Adelnia, F., et al., The Role of Muscle Perfusion in the Age-Associated Decline of Mitochondrial Function in Healthy Individuals. Front Physiol, 2019. 10: p. 427.
6. Stacy, M.R., et al., Application of BOLD Magnetic Resonance Imaging for Evaluating Regional Volumetric Foot Tissue Oxygenation: A Feasibility Study in Healthy Volunteers. Eur J Vasc Endovasc Surg, 2016. 51(5): p. 743-9.
7. Englund, E.K., et al., Intravoxel Incoherent Motion Magnetic Resonance Imaging in Skeletal Muscle: Review and Future Directions. J Magn Reson Imaging, 2021.
8. Reiter, D.A., et al., Parsimonious modeling of skeletal muscle perfusion: Connecting the stretched exponential and fractional Fickian diffusion. Magn Reson Med, 2021. 86(2): p. 1045-1057.
9. Cameron, D., et al., The effect of noise and lipid signals on determination of Gaussian and non-Gaussian diffusion parameters in skeletal muscle. NMR Biomed, 2017. 30(7).
10. Ivanov, K.P., M.K. Kalinina, and I. Levkovich Yu, Blood flow velocity in capillaries of brain and muscles and its physiological significance. Microvasc Res, 1981. 22(2): p. 143-55.
Figures