0072
Free Breathing 3D ASL Imaging of the Human Liver using Prospective Motion Correction: Preliminary Results1Fraunhofer MEVIS, Bremen, Germany, 2Faculty 1 (Physics/Electrical Engineering), University of Bremen, Bremen, Germany
Synopsis
Assessment of liver perfusion can yield valuable information about diseases like carcinoma and cirrhosis. Arterial spin labeling enables contrast-free measurements of liver perfusion with the drawback of increased motion sensitivity. Since timed breathing protocols prolong scan times, a novel prospective motion correction technique is presented, adjusting the initial saturation and image readout to the current position of the liver during the breathing cycle. The presented technique is compatible with highly suppressed signals, typically encountered in modern ASL imaging and achieved a significant decrease of cranial-caudal liver translation in acquired 3D GRASE volumes.
Introduction
Assessment of liver perfusion can yield valuable information about diseases like carcinoma and cirrhosis (1). Techniques like dynamic contrast-enhanced MR imaging (DCE-MRI) allow measurements of liver perfusion by application of exogenous contrast agents such as gadolinium (2)(3) but the application of contrast agents to patients with renal failure is controversial (4) and recent discussion of deposition of gadolinium-based contrast agents in brain increases the demand for a non-invasive alternative in clinical routine (5)(6)(7)(8).Non-invasive arterial spin labeling (ASL) MRI has the potential to overcome these limitations but is sensitive to motion of the liver due to the subject's respiration. Mitigation of motion-related artifacts by timed breathing protocols is effective but prolongs scan times. To this aim, we present a prospective cranial-caudal motion compensation technique which is compatible with the low signal levels, typically present in background suppressed 3D GRASE pCASL imaging.
Methods
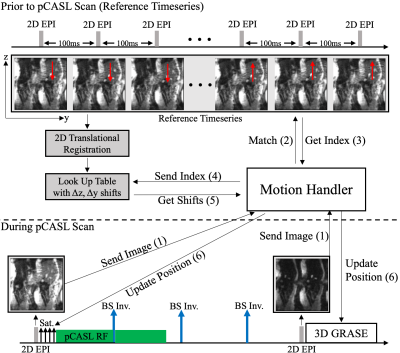
Prospective Motion CompensationThe correction of the large cranial-caudal liver shifts during free breathing pCASL experiments benefits from a prospective solution, which mitigates spin history artifacts. Therefore, positioning of initial saturation and 3D GRASE excitation regions is updated throughout the scan by inserting additional sagittal 2D EPI navigators into the pCASL sequence (cf. Fig. 1). Due to the high suppression of static tissue signal, a direct registration of navigator data is not possible. Instead, navigators are matched to a 2D EPI reference timeseries, which was acquired before the actual experiment. Cross-correlation based matching is possible due to residual fat signal in navigators and the reference series. Positioning is then updated based on corresponding entries from a lookup table. The lookup table is filled by image registration of the reference timeseries prior to the actual pCASL experiment.
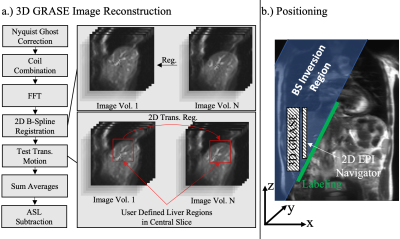
3D GRASE Image Reconstruction
The 3D GRASE image reconstruction pipeline is demonstrated in Fig. 2. After additional 2D elastic image registration ("Elastic"), a 2D translational registration of the upper liver boundary is performed and the z-component is analyzed, corresponding to residual cranial-caudal liver motion.
Experiments
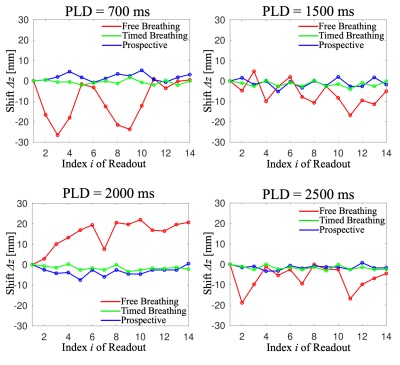
Initial measurements of a healthy volunteer were acquired at 3T (Siemens MAGNETOM Skyra, Siemens Healthineers, Erlangen). The subject provided written informed consent prior to scanning and the study was approved by the local ethics committee. As a reference, timed breathing pCASL scans were carried out (LD=2000 ms, post-labeling delays PLD=700 ms/1500 ms/2000 ms/2500 ms) with TR=10s ("Timed Breathing"). Experiments are repeated (TR=6s), but the subject was breathing freely throughout the scan (“Free Breathing”). Last, the same experiments are performed with prospective correction enabled (“Prospective”).
3D GRASE imaging parameters were: TE = 23.9ms, Bandwidth = 2264 HZ/Px, FoV = (250x208x40) mm3 with a matrix size of 48x40x8 pixels, yielding a voxel size of 5.2x5.2x5 mm3, seven averages, yielding a total acquisition time of 2:20 minutes for the timed breathing protocol and 1:24 for the free breathing/prospective protocol. In this work, a sagittal orientation of the 3D GRASE volume and saturation region is chosen to allow accurate estimation of residual cranial-caudal motion in acquired 3D GRASE data. A schematic representation of the positioning is demonstrated in Fig. 2b.
Results
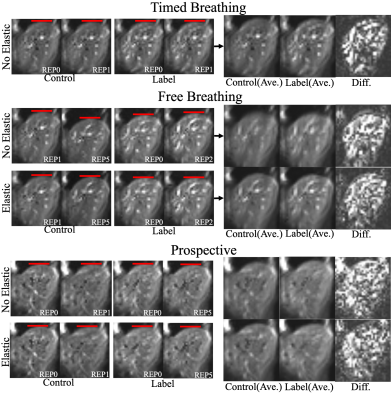
Fig. 3 shows calculated cranial-caudal z-trajectories with/without prospective motion compensation. Using the prospective correction, fluctuation of trajectories becomes less apparent when compared to the free breathing case, being comparable to the timed breathing protocol. Fig. 4 shows corresponding exemplary images from the experiment. Deviations in the liver's position are visibly reduced using the prospective solution. Elastic registration further reduces the misalignments.Discussion
The prospective motion compensation technique achieves reduction of cranial-caudal shifts in 3D GRASE images. By scanning additional subjects, the effectiveness of the presented technique will be further investigated. Future work will also investigate if external signals from respiratory cushions can be correlated with the reference series, removing the need for additional navigators during the pCASL experiment. The retrospective elastic correction allowed further reduction of residual motion in 3D GRASE volumes (cf. Fig. 4). By exploiting parallel imaging techniques, more partitions can be acquired within a single shot and future studies should investigate whether 3D elastic image registration is feasible, potentially further decreasing residual subtraction errors in perfusion-weighted images (cf. Fig. 4).Conclusion
In this work, we presented initial results from prospective motion compensation technique with additional 2D elastic image registration, which allowed to reduce cranial-caudal shifts of the liver in sagittal free breathing 3D GRASE pCASL. By acquiring additional datasets, a general measure of the techniques effectiveness will be derived, potentially paving the way to free breathing pCASL imaging of the human liver.Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) 446287025References
1. Do RKG, Rusinek H, Taouli B. Dynamic Contrast-Enhanced MR Imaging of the Liver: Current Status and Future Directions. Magn. Reson. Imaging Clin. N. Am. 2009
2. Aronhime S, Calcagno C, Jajamovich GH, et al. DCE-MRI of the liver: Effect of linear and nonlinear conversions on hepatic perfusion quantification and reproducibility. J. Magn. Reson. Imaging 2014
3. Jajamovich GH, Calcagno C, Dyvorne HA, Rusinek H, Taouli B. DCE-MRI of the Liver: Reconstruction of the Arterial Input Function Using a Low Dose Pre-Bolus Contrast Injection Hoffmann A-C, editor. PLoS One 2014
4. Schieda N, Blaichman JI, Costa AF, et al. Gadolinium-Based Contrast Agents in Kidney Disease: A Comprehensive Review and Clinical Practice Guideline Issued by the Canadian Association of Radiologists. Can. J. Kidney Heal. Dis. 2018
5. Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015
6. Radbruch A, Weberling LD, Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015
7. McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015
8. Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017
Figures