0070
Perfusion territory shifts in asymptomatic carotid artery stenosis measured by super-selective arterial spin labelling1Department of Diagnostic and Interventional Neuroradiology, School of Medicine, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 2TUM-Neuroimaging Center, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 3Philips Research, Hamburg, Germany, 4Philips Healthcare, Best, Netherlands, 5Department of Radiology, Neuroradiology and Minimal-Invasive Therapy, Klinikum Bogenhausen, Munich, Germany, 6Department of Diagnostic and Interventional Radiology, University Hospital Ulm, Ulm, Germany, 7Philips Healthcare, Hamburg, Germany
Synopsis
Vessel-selective imaging is promising to examine collateral blood supply in asymptomatic internal carotid artery stenosis (ICAS). Established modalities like digital subtraction angiography are invasive, not quantitative and associated with potential complication risks. A viable non-invasive alternative is super-selective arterial spin labelling, providing perfusion territories of individual arteries. We present data from seven asymptomatic ICAS patients and four age-matched healthy controls. We compared individual perfusion territory maps to an atlas of vascular territories and evaluated intra-hemispheric differences, allowing for quantitative assessment of stenotic mal-perfusion as well as compensatory collateral blood supply from the contralateral ICA.
Introduction
Cerebrovascular diseases are a major cause of death in developed countries.1 For example, internal carotid artery stenosis (ICAS) relates to an increased risk of stroke.1 While effective treatment is available, intervention risks complicate clinical decission making, particularly for asymptomatic ICAS.2 Evaluation of collateral blood crossflow has shown great potential to support treatment decisions.3,4 However, currently, the only clinically available imaging method is invasive digital subtraction angiography.A viable non-invasive alternative is super-selective arterial spin labelling (ssASL).5 ASL-based perfusion territory mapping showed promising results already6,7 and revealed perfusion territory shifts in symptomatic ICAS.8,9 However, for asymptomatic ICAS, the literature is controversial: while some studies show haemodynamic impairments within border zones between perfusion territories,10 others found no significant territorial differences for low-grade asymptomatic patients, but, on the other hand, steno-occluded asymptomatic patients’ haemodynamic was impaired.9 The aim of our study was, therefore, to establish quantitative measures for the evaluation of perfusion territory shifts and perfusion impairment in asymptomatic, high-grade ICAS based on ssASL. We hypothesized that asymptomatic ICAS may induce significant shifts of vascular territories in the frontal circulation.
Methods
Seven asymptomatic, patients with unilateral, high-grade ICAS (age (72.7±6.3) years, NASCET>70%) and four age-matched healthy controls (HC, age (70.0±6.2) years) underwent MRI on a 3T Philips Ingenia (Best, Netherlands). The imaging protocol included structural MRI, ssASL11 of both ICAs and non-selective pseudo-continuous ASL (pCASL) to measure cerebral blood flow (CBF, see Fig.1 for details and derived parameters). Quantitative analyses used SPM1212 and custom MATLAB programs. Based on ssASL data, individual perfusion territories were semi-automatically segmented (Fig.2C&D), normalized to MNI space, and averaged to calculate group-level probability maps (Fig.3).Individual perfusion territories were compared with an MNI-based atlas of vascular territories13 of the frontal circulation, i.e., of the anterior cerebral artery (ACA) and middle cerebral artery (MCA) (Fig.2E&F). Four parameters were evaluated. First, spatial overlap of individual and atlas territories by DICE coefficients. Second, the fractional volume of individual territories referenced to the whole brain volume. Third, grey matter (GM) CBF values (calculated from non-selective pCASL) within individual territories. Fourth, shifts of territories into the opposite hemisphere (fractional volume of contralateral perfusion territories in the ipsilateral hemisphere and vice versa). Paired t-tests were applied (p<0.05).
Results
Exemplary data of an ICAS patient show shifted perfusion territories (Fig.2). Frontal areas ipsilateral to the stenosis are supplied by the contralateral ICA (Fig.2C&D). The overlap of individual and atlas territories is larger in the contralateral hemisphere (Fig.2F). Group average perfusion territories show notable shifts of the contralateral anterior territory towards the ipsilateral hemisphere (Fig.3A&B), while HCs territories remain similar between hemispheres (Fig.3C&D).Statistical evaluations showed significantly larger overlap between individual and atlas territories in the contralateral vs. ipsilateral hemispheres (Fig.4A). Contralateral territories also comprised a significantly larger fraction of the whole brain volume (Fig.4B) and CBF values from quantitative pCASL were significantly increased in contralateral territories (Fig.4C). A clear but non-significant trend for shifts from contralateral to ipsilateral hemispheres was also found (Fig.4D). For HCs, all parameters were symmetrical between hemispheres. Comparisons of hemispheric differences revealed stronger lateralization for all parameters in ICAS compared to HCs (Table1).
Discussion
As hypothesized, our quantitative analyses indicate shifts of the cerebral blood supply in the frontal circulation. Changes in overlap between individual and atlas territories contra- and ipsilateral to the stenosis, respectively, indicate shifts of frontal perfusion territories. In addition, the volumes perfused by the contralateral ICA are larger. This resembles the situation in symptomatic ICAS, where increased collateral crossflow towards hypo-perfused territories was reported.8,9 Moreover, the asymmetric overlap indicates shifts at border zones between territories, which fits previous observations.10,14,15 Reduced CBF within vascular territories of the ipsilateral hemisphere agrees with previously reported hypo-perfusion in unilateral ICAS.10,16 Compared to HCs, territory shifts between hemispheres are clearly visible for ICAS patients. Some variability of territories in HCs is in line with literature.17,18 On group level, patients’ shifts were not statistically significant, most likely due to the low number of patients included. Increased collateral supply towards regions with lower CBF agrees with previously reported shifts of vascular border zones in our cohort14 and can be explained by regionally reduced cerebral perfusion pressure. The fact that other studies did not find territory shifts in asymptomatic ICAS9 may be explained by the inclusion of patients with lower grade (NASCET>50%) and bilateral stenosis.Compared to other ASL-based territory mapping techniques, that require sophisticated offline postprocessing,8,9,19 ssASL perfusion maps are readily available on the scanner console (Fig.2A&B).20,21 Moreover, ssASL allows great flexibility in labelling intracranial arteries.22 Clinical applicability is enhanced by automated ssASL labelling position identification,7,20,23 which can even be combined with non-invasive time-resolved selective ASL-angiography to gain additional collateral blood supply information.24
Conclusion
In conclusion, we demonstrated considerable shifts of vascular perfusion territories for high-grade asymptomatic ICAS using ssASL and segmentations of individual perfusion territories. Our results indicate increased collateral flow towards the frontal circulation of the ipsilateral hemisphere. Based on the easy applicable ssASL method, we introduced quantitative measures, which can improve diagnosis on a single subject level as well as detailed group level analyses. This could be especially useful for larger cohorts, such as expected from an ongoing ICAS study in our department.Acknowledgements
We acknowledge support by Dr.-Ing. Leonhard-Lorenz-Stiftung (grant SK 971/19) and the German research Foundation (DFG, grant PR 1039/6-1).References
1. Donahue MJ, Achten E, Cogswell PM, et al. Consensus statement on current and emerging methods for the diagnosis and evaluation of cerebrovascular disease. J Cereb Blood Flow Metab, 2018; 38(9):1391-1417.
2. Alberts MJ. Carotid stenting—why treating an artery may not treat the patient. JAMA neurology, 2015; 72(3):263-264.
3. Liebeskind DS. Collateral circulation. Stroke, 2003; 34(9):2279-2284.
4. Jung S, Wiest R, Gralla J, et al. Relevance of the cerebral collateral circulation in ischaemic stroke: time is brain, but collaterals set the pace. Swiss medical weekly, 2017; 147(w14538):w14538.
5. Helle M, Norris DG, Rüfer S, et al. Superselective pseudocontinuous arterial spin labeling. Magnetic Resonance in Medicine, 2010; 64(3):777-786.
6. Richter V, Helle M, van Osch M, et al. MR imaging of individual perfusion reorganization using superselective pseudocontinuous arterial spin-labeling in patients with complex extracranial steno-occlusive disease. American Journal of Neuroradiology, 2017; 38(4):703-711.
7. Kaczmarz S, Reichert M, Hernandez-Petzsche M, et al. Clinical application of ASL-based non-invasive perfusion territory mapping and time-resolved angiography in cerebrovascular diseases. ISMRM 2021, 2021;
8. van Laar PJ, Hendrikse J, Klijn CJ, et al. Symptomatic carotid artery occlusion: flow territories of major brain-feeding arteries. Radiology, 2007; 242(2):526-534.
9. Hartkamp NS, Petersen ET, Chappell MA, et al. Relationship between haemodynamic impairment and collateral blood flow in carotid artery disease. Journal of Cerebral Blood Flow & Metabolism, 2018; 38(11):2021-2032.
10. Kaczmarz S, Göttler J, Petr J, et al. Hemodynamic impairments within individual watershed areas in asymptomatic carotid artery stenosis by multimodal MRI. Journal of Cerebral Blood Flow & Metabolism, 2021; 41(2):380-396.
11. Helle M, Norris DG, Rüfer S, et al. Superselective pseudocontinuous arterial spin labeling. Magnetic resonance in medicine, 2010; 64(3):777-786.
12. Wellcome Centre for Human Neuroimaging. Statistical Parametric Mapping Software (SPM 12).
13. Liu C-F, Hsu J, Xu X, et al. Digital 3D Brain MRI Arterial Territories Atlas. bioRxiv, 2021; 2021.05.03.442478. (In Preparation).
14. Schmitzer L, Sollmann N, Kufer J, et al. Decreasing Spatial Variability of Individual Watershed Areas by Revascularization Therapy in Patients With High‐Grade Carotid Artery Stenosis. Journal of Magnetic Resonance Imaging, 2021;
15. Kaczmarz S, Griese V, Preibisch C, et al. Increased variability of watershed areas in patients with high-grade carotid stenosis. Neuroradiology, 2018; 60(3):311-323.
16. Göttler J, Kaczmarz S, Nuttall R, et al. The stronger one-sided relative hypoperfusion, the more pronounced ipsilateral spatial attentional bias in patients with asymptomatic carotid stenosis. Journal of Cerebral Blood Flow and Metabolism, 2020; 40(2):314-327.
17. van der Zwan A, Hillen B, Tulleken CA, et al. Variability of the territories of the major cerebral arteries. Journal of neurosurgery, 1992; 77(6):927-940.
18. van Laar PJ, Hendrikse J, Golay X, et al. In vivo flow territory mapping of major brain feeding arteries. Neuroimage, 2006; 29(1):136-144.
19. Gevers S, Bokkers R, Hendrikse J, et al. Robustness and reproducibility of flow territories defined by planning-free vessel-encoded pseudocontinuous arterial spin-labeling. American Journal of Neuroradiology, 2012; 33(2):E21-E25.
20. Sollmann N, Liebl H, Preibisch C, et al. Super-selective ASL and 4D ASL-based MR Angiography in a Patient with Moyamoya Disease. Clinical Neuroradiology, 2021; 31(2):515-519.
21. Hartkamp NS, Petersen ET, De Vis JB, et al. Mapping of cerebral perfusion territories using territorial arterial spin labeling: techniques and clinical application. NMR Biomed, 2013; 26(8):901-912.
22. Helle M, Rüfer S, van Osch MJ, et al. Superselective arterial spin labeling applied for flow territory mapping in various cerebrovascular diseases. Journal of Magnetic Resonance Imaging, 2013; 38(2):496-503.
23. Helle M, Wenzel F, van de Ven K, et al. Advanced automatic planning for super-selective arterial spin labeling flow territory mapping. Proc Int Soc Magn Reson Med, 2018; 26:302.
24. Togao O, Obara M, Helle M, et al. Vessel-selective 4D-MR angiography using super-selective pseudo-continuous arterial spin labeling may be a useful tool for assessing brain AVM hemodynamics. European Radiology, 2020; 30(12):6452-6463.
Figures
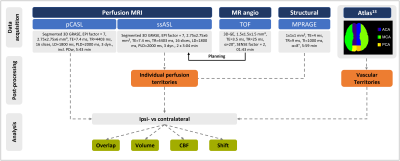
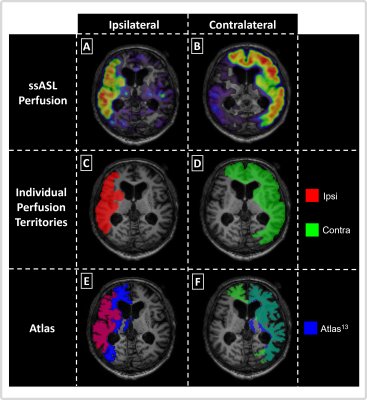
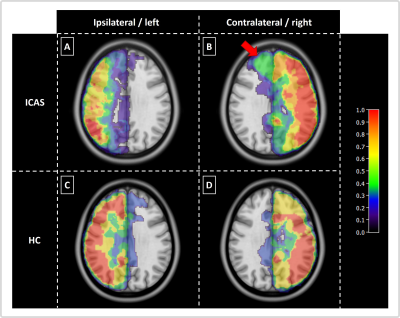
Figure 3: Group analysis: probability map of individual masks in MNI space. For comparison on group-level, individual masks where co-registered to MNI space and a probability map for perfusion territories calculated. For ICAS a shift of contralateral territories towards the ipsilateral hemisphere is clearly visible in the anterior circulation (indicated by red arrow, A&B), indicating collateral flow supply. For healthy controls (HC) perfusion territories are similar between hemispheres (C&D).
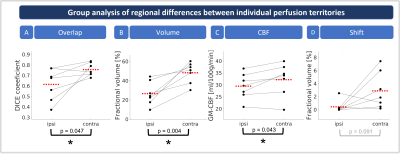

Table 1: Quantitative comparisons of ICAS patients and healthy controls (HC). Mean values are shown for the ipsi- and contralateral (ICAS) and right/left (HC) hemispheres. Significant differences are highlighted in green, bold print and marked by asterisks.