0067
Genetic and Environmental Contributions to Subcortical Gray Matter Microstructure and Volume in the Developing Brain1Department of Psychology, Yale University, New Haven, CT, United States, 2Tufts Medical Center, Boston, MA, United States
Synopsis
Using 9-10 year old monozygotic and dizygotic twins from the Adolescent Brain and Cognitive DevelopmentSM Study, we investigated the genetic and environmental contributions to microstructure (assessed using diffusion MRI and a restriction spectrum imaging model) and volume in nine subcortical gray matter regions. Heritability estimates range from 0.45 to 0.86 for microstructure, and 0.61 to 0.88 for volumes. Only the microstructure of the amygdala showed evidence of sensitivity to the shared environment.
INTRODUCTION
Human studies have shown that subcortical gray matter microstructure, assessed using diffusion MRI, is sensitive to development1, and stressful life events2. Using data from monozygotic (MZ) and dizygotic (DZ) twins, we aimed to determine the contributions of genetics, common environment, and unique environment to subcortical gray matter microstructure and volume in children.METHODS
The Adolescent Brain and Cognitive DevelopmentSM Study (ABCD Study®) recruited a nationally representative sample of nearly 12,000 children aged 9-10. This longitudinal study collects extensive cognitive, behavioral, genetic and imaging3 data, and includes a sub-study of 800 pairs of same-sex twin pairs4, enabling the assessment of the genetic and environmental influences of traits.To maximize data consistency, only baseline data (age 9-10) from twins scanned using a single MRI vendor were included in the analysis. Twin pairs were excluded if they had incomplete data, or if the structural and diffusion imaging data did not pass quality control, including a diffusion mean motion threshold of 1.5mm. Subjects with a history of brain injury, cerebral palsy, muscular dystrophy, multiple sclerosis, or substance abuse were excluded.
MRI data acquisition included diffusion-weighted imaging with b-values of 500 (6 directions), 1000 (15 directions), 2000 (15 directions), and 3000 (60 directions) s/mm2. Data preprocessing is described elsewhere5, and includes applying a restriction spectrum imaging6 model resulting in measures of the isotropic and directional restricted and hindered components of diffusion. The isotropic restricted component has been shown to be sensitive to tissue cellularity6. Data was residualized to account for the effects of age, sex, and site. Zygosity status was genetically determined.
The analysis was repeated based on the FreeSurfer-derived volumes of each subcortical structure, normalized to the total intracranial volume.
Structural Equation Modelling was used to assess the additive genetic (A), common environment (C), and unique environmental (E) contributions to tissue microstructure using and the OpenMx7 package in R (R version 3.6.1, R Core Team). A direct variance ACE model was used8.
RESULTS
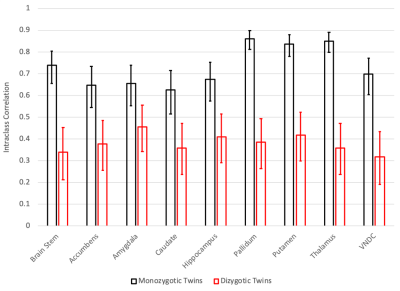
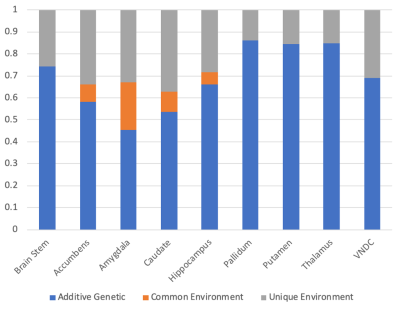
After applying exclusion criteria, 133 pairs of MZ twins and 153 pairs of DZ twins were included in the analysis of diffusion data. Figure 1 shows the intraclass correlations (ICCs) between twins for the restricted isotropic component of diffusion, while Figure 2 shows the calculated genetic, common environment, and unique environment contributions.All regions demonstrated significant (p<0.05) additive genetic contributions, ranging from 0.45 in the amygdala to 0.86 in the pallidum. Only the amygdala showed evidence of a contribution from the common environment (p=0.057). Unique environment contributed between 0.14 (pallidum) and 0.37 (caudate) of the variance.
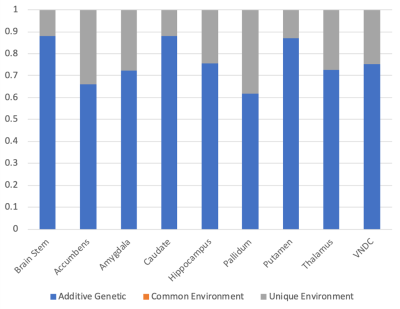
148 pairs of MZ twins and 190 pairs of DZ twins were included in the analysis of the volumetric data. Figure 3 shows the calculated additive genetic, common, and unique environment contributions to subcortical volumes. The genetic contributions ranged from 0.61 (pallidum) to 0.88 (brain stem, and caudate). No regions showed evidence of the impact of common environment.
DISCUSSION
While diffusion MRI is commonly using to assess white matter development, few studies have applied it to subcortical gray matter development9-11. The ABCD Study is the largest imaging study of development in the US with over 11,000 youth, but the relatively small number of twin pairs enrolled is a limitation. In general, the uncertainties in the estimates of the contributions are large. However, in all regions, the genetic contributions to subcortical microstructure are statistically significant, and in some cases very high, for example in the pallidum (0.86, 95% CI of 0.82-0.89), thalamus (0.85, 95% CI of 0.80-0.88), and putamen (0.84, 95% CI of 0.80-0.88).The amygdala is notable in its relatively low genetic contribution (0.45, 95% CI of 0.21-0.71), and in having evidence of a contribution from the common environment (0.22, 95% CI –0.01-0.42), consistent with prior animal imaging studies12.
The calculated unique environment contribution accounts for all the remaining variance between twin pairs, including measurement uncertainties. Comparing individuals at baseline and at year 2 follow-up revealed ICC within individuals to be similar to those observed between the MZ twins at baseline. We speculate that measurement error accounts for a substantial fraction of the unique environment variance, and that the genetic contribution calculated in this study should be interpreted as a lower limit of the true genetic contribution to microstructure.
The genetic influence on the volumes of subcortical structures were consistently higher than those reported in a previous study of adult twins13. This could represent methodological improvements in data acquisition or analysis, or an increasing influence of the unique environment during adulthood.
CONCLUSION
We believe that the current study is the first to investigate the genetic and environmental contributions to subcortical gray matter during childhood. We find that both subcortical gray matter microstructure and volume are highly heritable. Only the amygdala microstructure showed a contribution of the common environment. In this study, we only included baseline data at age 9-10, and these contributions may evolve over time. Some latent genetic factors may become important during puberty, while twins may experience diverging environments as they approach adulthood. The longitudinal nature of the study will enable assessment of the evolving influence of genetic and environmental factors during the critical neurodevelopment period of adolescence.Acknowledgements
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
The ABCD data repository grows and changes over time. The ABCD data used in this report came from Curated Data Release 4.0, http://dx.doi.org/10.15154/1523041
References
1. Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage 2008;40(3):1044-1055.
2. Conley MI, Skalaban LJ, Rapuano KM, Gonzalez R, Laird AR, Dick AS, Sutherland MT, Watts R, Casey BJ. Altered hippocampal microstructure and function in children who experienced Hurricane Irma. Dev Psychobiol 2021;63(5):864-877.
3. Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich MT, Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, Chaarani B, Mejia MH, Hagler DJ, Jr., Daniela Cornejo M, Sicat CS, Harms MP, Dosenbach NUF, Rosenberg M, Earl E, Bartsch H, Watts R, Polimeni JR, Kuperman JM, Fair DA, Dale AM, Workgroup AIA. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci 2018;32:43-54.
4. Iacono WG, Heath AC, Hewitt JK, Neale MC, Banich MT, Luciana MM, Madden PA, Barch DM, Bjork JM. The utility of twins in developmental cognitive neuroscience research: How twins strengthen the ABCD research design. Dev Cogn Neurosci 2018;32:30-42.
5. Hagler DJ, Jr., Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, Sutherland MT, Casey BJ, Barch DM, Harms MP, Watts R, Bjork JM, Garavan HP, Hilmer L, Pung CJ, Sicat CS, Kuperman J, Bartsch H, Xue F, Heitzeg MM, Laird AR, Trinh TT, Gonzalez R, Tapert SF, Riedel MC, Squeglia LM, Hyde LW, Rosenberg MD, Earl EA, Howlett KD, Baker FC, Soules M, Diaz J, de Leon OR, Thompson WK, Neale MC, Herting M, Sowell ER, Alvarez RP, Hawes SW, Sanchez M, Bodurka J, Breslin FJ, Morris AS, Paulus MP, Simmons WK, Polimeni JR, van der Kouwe A, Nencka AS, Gray KM, Pierpaoli C, Matochik JA, Noronha A, Aklin WM, Conway K, Glantz M, Hoffman E, Little R, Lopez M, Pariyadath V, Weiss SR, Wolff-Hughes DL, DelCarmen-Wiggins R, Feldstein Ewing SW, Miranda-Dominguez O, Nagel BJ, Perrone AJ, Sturgeon DT, Goldstone A, Pfefferbaum A, Pohl KM, Prouty D, Uban K, Bookheimer SY, Dapretto M, Galvan A, Bagot K, Giedd J, Infante MA, Jacobus J, Patrick K, Shilling PD, Desikan R, Li Y, Sugrue L, Banich MT, Friedman N, Hewitt JK, Hopfer C, Sakai J, Tanabe J, Cottler LB, Nixon SJ, Chang L, Cloak C, Ernst T, Reeves G, Kennedy DN, Heeringa S, Peltier S, Schulenberg J, Sripada C, Zucker RA, Iacono WG, Luciana M, Calabro FJ, Clark DB, Lewis DA, Luna B, Schirda C, Brima T, Foxe JJ, Freedman EG, Mruzek DW, Mason MJ, Huber R, McGlade E, Prescot A, Renshaw PF, Yurgelun-Todd DA, Allgaier NA, Dumas JA, Ivanova M, Potter A, Florsheim P, Larson C, Lisdahl K, Charness ME, Fuemmeler B, Hettema JM, Maes HH, Steinberg J, Anokhin AP, Glaser P, Heath AC, Madden PA, Baskin-Sommers A, Constable RT, Grant SJ, Dowling GJ, Brown SA, Jernigan TL, Dale AM. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage 2019;202:116091.
6. White NS, Leergaard TB, D'Arceuil H, Bjaalie JG, Dale AM. Probing tissue microstructure with restriction spectrum imaging: Histological and theoretical validation. Human brain mapping 2013;34(2):327-346.
7. Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika 2011;76(2):306-317.
8. Verhulst B, Prom-Wormley E, Keller M, Medland S, Neale MC. Type I Error Rates and Parameter Bias in Multivariate Behavioral Genetic Models. Behavior genetics 2019;49(1):99-111.
9. Mah A, Geeraert B, Lebel C. Detailing neuroanatomical development in late childhood and early adolescence using NODDI. PloS one 2017;12(8):e0182340.
10. Eaton-Rosen Z, Melbourne A, Orasanu E, Cardoso MJ, Modat M, Bainbridge A, Kendall GS, Robertson NJ, Marlow N, Ourselin S. Longitudinal measurement of the developing grey matter in preterm subjects using multi-modal MRI. NeuroImage 2015;111:580-589.
11. Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. NeuroImage 2005;26(4):1164-1173.
12. Ding AY, Li Q, Zhou IY, Ma SJ, Tong G, McAlonan GM, Wu EX. MR diffusion tensor imaging detects rapid microstructural changes in amygdala and hippocampus following fear conditioning in mice. PloS one 2013;8(1):e51704.
13. Gillespie NA, Neale MC, Hagler DJ, Jr., Eyler LT, Fennema-Notestine C, Franz CE, Lyons MJ, McEvoy LK, Dale AM, Panizzon MS, Kremen WS. Genetic and environmental influences on mean diffusivity and volume in subcortical brain regions. Human brain mapping 2017;38(5):2589-2598.
Figures