0065
Quantification of sulcal emergence in the fetal brain: hemispheric asymmetry and sex difference
Hyuk Jin Yun1, Hyun Ju Lee2, Joo Young Lee2, Tomo Tarui3, Caitlin K. Rollins1, Cynthia M. Ortinau4, Henry A. Feldman1, P. Ellen Grant1, and Kiho Im1
1Boston Children's Hospital, Boston, MA, United States, 2Hanyang University, Seoul, Korea, Republic of, 3Tufts Medical Center, Boston, MA, United States, 4Washington University in St. Louis, St. Louis, MO, United States
1Boston Children's Hospital, Boston, MA, United States, 2Hanyang University, Seoul, Korea, Republic of, 3Tufts Medical Center, Boston, MA, United States, 4Washington University in St. Louis, St. Louis, MO, United States
Synopsis
Sulcal emergence is important to assess normality of early fetal brain development. Thus, we proposed a quantitative approach to build an accurate timetable of sulcal emergence. Using a large sample of fetuses, we automatically extracted cortical surfaces and detected presence and absences of each sulcus. By fitting a logistic curve to sulcal presence/absence, the timing and inter-subject variability of sulcal emergence were estimated. We also found hemispheric asymmetry and sex difference in the timing and variability of sulcal emergence. Our quantitative timetable concurs with previous reports but provides more precise information and allow statistical comparison.
Introduction
In the fetal brain, the timing of sulcal emergence has been considered a key marker for evaluating the normality of cortical development in numerous studies1–5. Sulcal emergence shows regionally different patterns that may be related to dynamic spatiotemporal patterns of gene expression6–9. Since gene expression patterns are different across typical individuals, sulcal emergence may also have inter-subject variability. The timing and inter-subject variability of sulcal emergence in typically developing (TD) fetuses can provide crucial information to define normal range of fetal brain development and can be used as a reference to detect potential abnormalities in early brain development.We aimed to quantitatively measure the timing of sulcal emergence and its inter-subject variability. We also provided a statistical approach to compare difference in timing and its variability between groups. Here, we statistically investigated hemispheric asymmetry and sex difference in the timing and variability of sulcal emergence.
Methods
A total of 89 TD fetuses were included in this study (gestational weeks [GW]: 26.6 ± 3.6 [mean ± standard deviation (SD)], sex: 47/40/2 [male/female/unknown]). We used our automatic pipeline for fetal brain MRI processing and cortical surface reconstruction10. Fetal sulci in the cortical surfaces were anatomically assigned to 19 labels using spatial and temporal information of fetal gyrification from multiple fetal templates from 23 to 33GW10. The fetal sulcal labels and abbreviations are shown in Figure 1. To estimate the timing and variability of sulcal emergence, the presence or absence of sulcal labels in each fetus was counted as binary variables. In each sulcus, we modeled a binary logistic curve to quantify the relationship between GW and the binary variables that represents the probability of sulcal label presence by GW. We estimated sulcal emergence timing using GW at 0.5 of probability in the curve (t50). We also calculated interquartile range (IQR) of GWs between probability at 0.25 (t25) and 0.75 (t75) as the inter-subject variability of sulcal emergence (Figure 2A). To analyze hemispheric asymmetry and sex difference in the timing of sulcal emergence, we statistically compared two logistic curves modeled in each hemisphere (Figure 2B). Standard error (SE) of t50 in a group (hemisphere or sex) were calculated and the statistical difference in the timings were calculated by z transformation of t50s and SEs. Comparison of inter-subject variability was also performed by substituting t50 for IQR.Results
Table 1 shows the timing and variability of sulcal emergence. Emergence of the STS and CS occurred first as early as 23.67 and 24.10 GWs, respectively. The bilateral SFS, IFS, PreCS, PostCS, IPS, and OrbS emerged between 24.9 and 27 GWs. The bilateral MFS and OTS emerged after 27 GW. The inter-subject variability of sulcal emergence were variant across sulci. IQRs of left CS and PreCS were less than 1 week. Extremely small IQR were also found in the left IPS and right SFS. In contrast, the bilateral OTS, left IFS, right Post CS, right IFS, right ITS, right SPS had large IQR over two weeks. Since the SF, ColS, CingS, CalS, and POS emerge before 22 GW in all fetal brains, their binary logistic curves were not modeled, and we exclude them in the analysis.Statistically significant earlier sulcal emergence timing in the right hemisphere was found in the CS (p = 0.042) and OTS (p = 0.010) (Table 1). Significantly smaller variability in the left hemisphere was found in the PostCS (p = 0.045). For sex difference (Table 2), the sulcal emergence timing of males was significantly earlier than that of females in the left PreCS (p < 0.001), left MFS (p = 0.023), left IFS (p = 0.005), and right ITS (p = 0.040). Significantly larger variability of sulcal emergence timing in male fetuses was found in the bilateral IPS (p = 0.003 [left] and 0.013 [right]).
Discussion
This study is the first to quantify sulcal emergence in a large sample of TD fetuses. The sequence of emergence timing estimated by our quantitative approach indicates that the sulcal emergence occurred from central to peripheral regions. This finding concurs with the prior studies1,2,11–13 and may be related the temporo-parieto-occipital pattern of brain maturation. The majority of sulci showed 1-2 weeks of inter-subject variability of sulcal emergence timing, which is supported by previous studies with visual inspection1,2. While the visual inspection studies reported uniform variabilities across sulci, our quantitative approach found the regionally diverse variability that may be associated with different temporal variability of gene expression in each region.Furthermore, we found left CS and OTS emerged earlier than right, which is supported by previous studies on hemispheric asymmetry of brain structures14–17. Sex difference in emergence timing and its variability reported in this study may be associated with sex difference in regional volumes in the fetal brain17.
In conclusion, our approach provided a quantitative timetable of sulcal emergence that could be a reference to assess normality of fetal gyrification, and to allow further statistical analysis of fetal gyrification. Future studies will include enrichment for numerous factors that need to be explored including race, ethnicity, socioeconomic status and maternal education.
Acknowledgements
No acknowledgement found.References
1. Chi, J. G., Dooling, E. C. & Gilles, F. H. Gyral development of the human brain. Ann. Neurol. 1, 86–93 (1977). 2. Garel, C. et al. Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. AJNR Am. J. Neuroradiol. 22, 184–189 (2001). 3. Nishikuni, K. & Ribas, G. C. Study of fetal and postnatal morphological development of the brain sulci: Laboratory investigation. J. Neurosurg. Pediatr. 11, 1–11 (2013). 4. Levine, D. & Barnes, P. D. Cortical Maturation in Normal and Abnormal Fetuses as Assessed with Prenatal MR Imaging. Radiology 210, 751–758 (1999). 5. Batchelor, P. G. et al. Measures of folding applied to the development of the human fetal brain. IEEE Trans. Med. Imaging 21, 953–965 (2002). 6. Bystron, I., Blakemore, C. & Rakic, P. Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 9, 110–122 (2008). 7. Kang, H. J. et al. Spatio-temporal transcriptome of the human brain. Nature 478, 483–489 (2011). 8. Miller, J. A. et al. Transcriptional landscape of the prenatal human brain. Nature 508, 199–206 (2014). 9. Pletikos, M. et al. Temporal Specification and Bilaterality of Human Neocortical Topographic Gene Expression. Neuron 81, 321–332 (2014). 10. Yun, H. J. et al. Automatic labeling of cortical sulci for the human fetal brain based on spatio-temporal information of gyrification. NeuroImage 188, 473–482 (2019). 11. Habas, P. A. et al. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb. Cortex N. Y. N 1991 22, 13–25 (2012). 12. Yun, H. J. et al. Temporal Patterns of Emergence and Spatial Distribution of Sulcal Pits During Fetal Life. Cereb. Cortex 30, 4257–4268 (2020). 13. Clouchoux, C. et al. Quantitative in vivo MRI measurement of cortical development in the fetus. Brain Struct. Funct. 217, 127–139 (2012). 14. Ratnarajah, N. et al. Structural connectivity asymmetry in the neonatal brain. NeuroImage 75, 187–194 (2013). 15. Cykowski, M. D. et al. The central sulcus: an observer-independent characterization of sulcal landmarks and depth asymmetry. Cereb. Cortex N. Y. N 1991 18, 1999–2009 (2008). 16. Zilles, K. et al. Quantitative analysis of sulci in the human cerebral cortex: Development, regional heterogeneity, gender difference, asymmetry, intersubject variability and cortical architecture. Hum. Brain Mapp. 5, 218–221 (1997). 17. Vasung, L. et al. Quantitative In vivo MRI Assessment of Structural Asymmetries and Sexual Dimorphism of Transient Fetal Compartments in the Human Brain. Cereb. Cortex (2020) doi:10.1093/cercor/bhz200.Figures
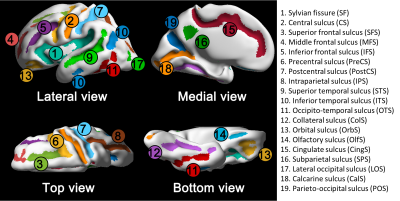
Figure 1. Primary sulcal labels. Colored regions indicate 19
primary sulci. Colored regions indicate 19 primary sulci.
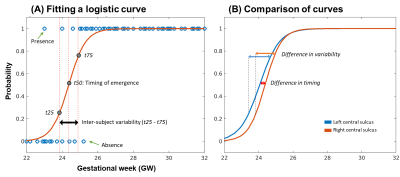
Figure 2. Estimation
and comparison of sulcal emergence.
(A) By fitting a logistic curve, emergence timing and its inter-subject
variability in the left CS were estimated. (B) Hemispheric asymmetry of sulcal
emergence was examined between left and right CS.
Table 1. Timing and inter-subject variability of sulcal emergence and
statistical results of hemispheric asymmetry. Positive z value indicates
that left sulcus has earlier timing or larger variability. *: p
< 0.05
Table 2. Sex difference in sulcal emergence timing and its
variability. Positive z value indicates
that sulcus of male fetuses has earlier timing or larger variability. *:
p < 0.05
DOI: https://doi.org/10.58530/2022/0065