0061
Numerically Optimized Magnet Designs for a Portable, Low-Field Neonatal MRI Brain Scanner1Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States, 2A. A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Division of Newborn Medicine, Department of Pediatrics, Massachusetts General Hospital, Boston, MA, United States
Synopsis
Point-of-care MRI scanners sited in the neonatal intensive care unit (NICU) can provide immense benefits for diagnosing and monitoring neonatal brain injury. While NICU sited systems can address some of the transport concerns, a truly portable “isolette-side” imager with a neonate-specific design could further improve imaging access. We present a neonate-specific lightweight MRI permanent magnet design for use at the bedside in the NICU. We examine two magnet designs demonstrating the feasibility of realizing a sufficiently homogeneous main magnet with mean B0 of 125mT weighing under 15kg, both with and without a built-in gradient of 10mT/m.
Introduction
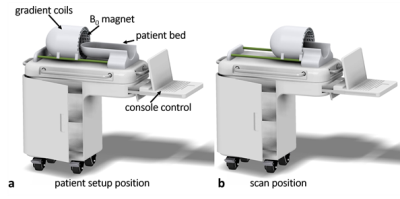
The neonatal brain is particularly vulnerable to a wide variety of insults, including birth asphyxia or Hypoxic-Ischemic Encephalopathy (HIE), meningitis, and stroke, each having the potential to lead to lifelong neurological deficits or premature death. HIE occurs in 1 to 8 per 1000 live births in developed countries, and up to 2.6% in resource-limited settings1,2 and causes 23% of neonatal deaths3. The pattern of HIE and resulting hypoxic-ischemic injury (HII) is evaluated most sensitively by diffusion-weighted MRI; however, patients are often too unstable to be moved to a traditional scanner. Currently, there are truly portable imaging systems that may be wheeled to the isolette4, and there are systems designed specifically for the neonate5,6, but there is no system that combines both features. A neonatal, bedside MRI device would allow for expedited diagnosis and intervention and may assist with prognostication. We propose a close-fitting whole-head MRI magnet constructed from NdFeB blocks, a design we have demonstrated to be effective for adult brain imaging7. Gradient coils would be positioned external to the magnet, and RF coils internal to it (Figure 1). Here, we present two numerically optimized magnet designs for such a device: one with maximally uniform field, and one with a built-in gradient for read-out encoding. The built-in gradient eliminates the need for the standard read-out gradient system, reducing acoustic noise, power needs, heating, size, and cost. Each magnet array is composed of NdFeB magnet blocks in a Halbach-bulb shape, with the size and orientation of each block optimized to achieve the desired field over a 14cm spherical ROI. The results demonstrate that a lightweight (<15kg), whole-brain magnet for neonates with B0=125mT, with and without built-in gradient of 10mT/m, is achievable with acceptable homogeneity using the proposed design.Methods
In theory, spatially uniform magnetic fields may be achieved using an ideal Halbach magnet array8,9; however, these may only be approximated in practice. Previously, we have demonstrated a genetic algorithm for optimizing the magnetic material of blocks in a truncated cylindrical Halbach array10, and an interior point method for optimizing block size and orientation in a helmet shaped Halbach array11. Here, we adapt this interior point method to optimize the size and orientation of magnetic blocks in a Halbach-bulb shape to design two magnet arrays, each composed of 184 blocks. The first design minimizes the absolute range of B0 magnitude over a 14cm spherical ROI. The second minimizes maximum variation from a desired 10mT/m gradient over the same ROI. Both optimizations constrain the mean B0 to be at least 125mT, and the magnetic dipole moment magnitude of each block to not exceed that of a 1”x1”x1” block of N52 magnet material. This optimization was performed in MATLAB using the previously applied interior point method11, modeling each magnet block using the first, third, and fifth multipole components. The desired magnetic moment of each block was then converted into a corresponding block volume and used to generate a design of 184 discrete N52 magnetic blocks. Finally, the generated design was evaluated using the MATLAB model used in the optimization and BiotSavart Magnetic Field Calculator Software12, which each consider multipole terms of up to fifth order.Results
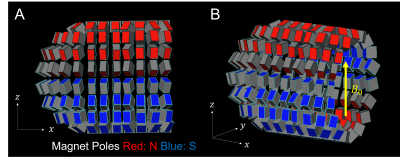
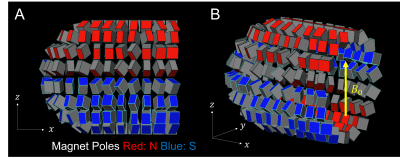
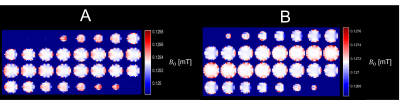
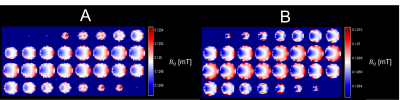
The optimized magnet designs for the uniform-field design and built-in gradient design are shown in Figures 2 and 3 respectively. These designs both have maximum linear dimensions of (x=30cm, y=25cm, z=25cm), with the estimated weight of the uniform-field design (including only the magnets themselves) being 13kg, and of the built-in gradient design being 14kg. The simulated mean B0 values for the uniform-field design were: 125.3mT, and 127.2mT, as computed with the MATLAB model and BiotSavart respectively, with corresponding field ranges of 0.62mT and 1.73mT over the ROI. The simulated mean B0 for the built-in gradient design were 125.0mT and 126.7mT, as computed with the MATLAB model and BiotSavart respectively. The error fields (the difference between the simulated field and the target field) have ranges of 2.59mT and 3.00mT respectively over the ROI. Figures 4 and 5 show the field maps for the designs.Discussion
Here, we design two numerically optimized magnets for a novel point-of-care neonatal brain MRI scanner, one with a maximally uniform field with a mean value of 125mT and one with a built-in gradient of 10mT/m, with the intent to allow diffusion-weighted imaging of neonatal brains. Using both an in-house MATLAB method and BiotSavart, it was found that these magnets achieved these aims with acceptable variations (although use of shim material will be needed). There were significant discrepancies between the results found using each method likely attributable to the differences in resolution used in each computation. Of note, these simulations did not consider interactions between the magnets, which is the immediate next step in validating these designs. Subsequently, this magnet will be constructed from a physically realizable combination of magnetic blocks of different size and material attached to a magnet former.Acknowledgements
We acknowledge Patrick C. McDaniel for developing the simulation and optimization code and thank Martin Hurlimann for insightful discussion on future directions. Funding from NIH NICHD R01HD104649.
References
1. Douglas-Escobar M, Weiss MD. Hypoxic-Ischemic Encephalopathy A Review for the Clinician. JAMA Pediatr. 2015;169:397–403 doi: 10.1001/jamapediatrics.2014.3269.
2. Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010;375:1969–1987 doi: 10.1016/S0140-6736(10)60549-1.
3. Oorschot DE, Sizemore RJ, Amer AR. Treatment of Neonatal Hypoxic-Ischemic Encephalopathy with Erythropoietin Alone, and Erythropoietin Combined with Hypothermia: History, Current Status, and Future Research. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21041487.
4. Deoni, SC, Bruchhage, MM, Beauchemin, J, Volpe, A, D'Sa, V, Huentelman, M, Williams, SC, 2021. Accessible Pediatric Neuroimaging using a Low Field Strength MRI Scanner. NeuroImage, p.118273.
5. Merhar SL, Tkach JA, Woods JC, et al. Neonatal imaging using an on-site small footprint MR scanner. Pediatr. Radiol. 2017;47:1001–1011 doi: 10.1007/s00247-017-3855-4.
6. Griffiths PD, Jarvis D, Armstrong L, et al. Initial experience of an investigational 3T MR scanner designed for use on neonatal wards. Eur. Radiol. 2018;28:4438–4446 doi: 10.1007/s00330-018-5357-7.
7. Cooley CZ, Stockmann JP, Wald LL. A portable brain MRI scanner based on a 72 mT, 35 kg "Halbach-bulb" magnet and external gradient coil. In: ISMRM. 2021; p. 0559
8. Halbach K. Design of permanent multipole magnets with oriented rareearth cobalt material. Nucl. Instruments Methods 1980;169:1–10.
9. Leupold H, Potenziani II E. Novel High-Field Permanent-Magnet FluxSources. IEEE Trans. Magn. 1987;MAG-23:3628–3629.
10. Cooley CZ, Haskell MW, Cauley SF, Sappo C, Lapierre CD, Ha CG,Stockmann JP, Wald LL. Design of Sparse Halbach Magnet Arrays for Portable MRI Using a Genetic Algorithm. IEEE Trans.Magn. 2017.
11. McDaniel PD, Cooley CZ, Stockmann JP, Wald LL. Numerically optimized design for a low-cost, lightweight 86mT whole-brain magnet. In: ISMRM. 2019; p. 1466
12. BiotSavart Magnetic Field Calculator, M. W. Reynolds, Ripplon Software Inc., Burnaby, BC, Canada. http://www.ripplon.com
Figures