0060
Fast 3D acquisition of wave displacement in vivo for low-field MRE: application in the human forearm1Center for Adaptable MRI Technology (AMT Center), Department of Biomedical Engineering, University of Basel, Allschwil, Switzerland
Synopsis
We describe a method to capture wave displacement in vivo in the human forearm for magnetic resonance elastography (MRE) at low magnetic field (0.1 T). Taking advantage of the inherently low spatial frequency nature of propagating waves, the proposed method samples a very low fraction (10%) of the 3D k-space, combined with efficient motion-encoding, processing schemes, and an optimized RF quadrature volume coil. For the first time, acquisitions are demonstrated in humans at a field below 1.5 T within only a few minutes (1-3 min).
Introduction
MR elastography (MRE) allows to quantify the stiffness of living tissue non-invasively1,2. Recently, low-field (LF) MRI research has regained popularity due to numerous advantages that can be naturally gained from operating at weaker magnetic fields3. Added with MRE capability, LF-MRI could provide wide accessibility to remote palpation with MRI, relevant in a number of pathologies such as chronic liver diseases, cancer or musculoskeletal disorders, yet at a reduced cost and with the benefit of increased safety and immunity to susceptibility issues.Performing LF-MRE is however non-trivial due to numerous challenges: low magnetic fields provide low net magnetizations, and MRE typically requires long echo times to accommodate motion-encoding gradients, thus further reducing the available signal. Translating accelerated high-field MRE sequences (mostly 2D) to low field is difficult due to the lower efficiency of such sequences with an impeded sensitivity, hence requiring averaging that translates in longer acquisition times. As a result, MRE at fields below 1.5 T has been performed only in ex vivo samples4–6, phantoms7,8 and small animals8–10.
Our approach for LF-MRE consists in accelerating a 3D cartesian motion-sensitized sequence by sampling only 10% of k-space along with a custom, optimized RF coil. This sampling scheme focuses on retrieving low spatial frequency information typical of MRE shear waves displacement, generally with vibration in the order of 30-150 Hz in humans.
The proposed approach is validated in a phantom and further applied in vivo in the human forearm, taking only 1 minute for a single vibration timepoint, and adding <30 s scan time for any additional timepoint (e.g. total of <3 min for 5 timepoints).
Methods
A resistive, biplanar 0.1 T scanner for extremities (EAR54L, Drusch & Cie, France) was used11 with a custom, biplanar quadrature RF coil12. A custom vibration system13 generated pressure waves conveyed via a waveguide (resonant frequencies 49, 89, and 129 Hz) to a soft PET flask acting as a transducer (Fig1).A rectangular phantom was made of bi-component silicone Eurosil4 (Schouten Syntec, Netherlands) with the addition of a softener (S) to create two distinct compartments (A and B in Fig3 with S-ratio 1.5 and 2.5, respectively)13. MRE scans were performed on the phantom and in vivo in the forearms of two healthy volunteers. A double-echo-steady-state sequence was further acquired to capture the anatomy of the subjects.
The motion-sensitized sequence was implemented on a Cameleon3 spectrometer (RS2D, France). Based on 3D gradient-echo with cartesian sampling, the sequence used bipolar trapezoidal motion-encoding-gradients (MEG) applied along one single direction (PE2 in Fig1). Only the central 10% of all the phase-encoding steps were acquired (Fig2). Two datasets with opposite MEG polarities were acquired consecutively for subtraction and removal of non-motion-related phase accumulation while doubling the encoding efficiency14,15. In addition, a reference dataset was acquired with MEGs turned on and vibration off, to remove the effects of imperfect MEG balancing. A single reference was sufficient for all encoded timepoints. The acquisition parameters are summarized in Fig2. Raw data were processed separately for each coil channel before they were combined for further analyses.
Wave profiles were extracted from 9 (manually positioned) segments on the processed phase images, and shear stiffness $$$μ$$$ was calculated from the estimated half-wavelength on each profile16–22 as:
$$\mu = \rho (\lambda f_{vib})^2$$
Mean and standard deviation values were calculated across all profiles.
Results
In Fig3a, the processed phase maps exhibit the encoded wave propagation in the phantom using a 49-Hz vibration. The two phantom compartments exhibit a different wavelength and thus different shear stiffness (57.1±4.2 mm and 7.9±1.1 kPa for compartment A, and 43.4±2.6 mm and 4.5±0.5 kPa for compartment B), as expected (Fig3b).Fig4 shows the phase-encoded wave displacement in vivo in two volunteers. The anatomical overlay (Fig4A) shows that the wave propagates in the forearm muscles. The wave profiles and corresponding $$$μ$$$ estimation (89 Hz: 32.9±3.9 mm, 8.6±0.5 kPa; 129 Hz: 38.9±0.0 mm, 25.2±0.0 kPa) show an increased $$$μ$$$ at higher frequencies, as expected from the dispersive behavior of the muscle23.
Discussion
Due to the inherently low frequency nature of mechanical waves propagating in MRE, our results indicate that 10%-sampling of k-space combined with tailored acquisition and processing schemes allows to efficiently capture shear waves propagation, both in phantoms and in vivo. The phase-encoded wave displacement images exhibit the expected propagation pattern and enable wave profile extraction for wavelength and shear stiffness estimation. Unlike 2D scans, the isotropic, 3D acquisition provides a continuous 3D dataset of wave propagation, which can be visualized and processed for any orientation.Conclusions
The proposed k-space sampling and processing approach greatly accelerate the encoding of shear waves by using a 3D cartesian gradient-echo sequence. For the first time, acquisitions for MRE were successfully performed in humans in vivo at low magnetic field (0.1 T). A single wave acquisition took less than 30 s in vivo, for a total scan of 1-3 min to obtain 1-5 vibration timepoints, opening perspectives for the democratization of LF-MRE in small-footprint compact scanners.Acknowledgements
Swiss National Science Foundation Grants 170575, 198905, 186861.References
1. Muthupillai, R. et al. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science (80-. ). 269, 1854–1857 (1995).
2. Plewes, D. B., Betty, I., Urchuk, S. N. & Soutar, I. Visualizing tissue compliance with MR imaging. J. Magn. Reson. Imaging 5, 733–738 (1995).
3. Sarracanie, M. & Salameh, N. Low-Field MRI: How Low Can We Go? A Fresh View on an Old Debate. Front. Phys. 8, 1–14 (2020).
4. Zampini, M. A., Guidetti, M., Royston, T. J. & Klatt, D. Measuring viscoelastic parameters in Magnetic Resonance Elastography: a comparison at high and low magnetic field intensity. J. Mech. Behav. Biomed. Mater. 120, 104587 (2021).
5. Braun, J. et al. A compact 0.5 T MR elastography device and its application for studying viscoelasticity changes in biological tissues during progressive formalin fixation. Magn. Reson. Med. 79, 470–478 (2018).
6. Ipek-Ugay, S. et al. Tabletop magnetic resonance elastography for the measurement of viscoelastic parameters of small tissue samples. J. Magn. Reson. 251, 13–18 (2015).
7. Salameh, N., Sarracanie, M., Armstrong, B. D., Rosen, M. S. & Comment, A. Overhauser-enhanced magnetic resonance elastography. NMR Biomed. 29, 607–613 (2016).
8. Vappou, J. et al. Dynamic viscoelastic shear properties of soft matter by magnetic resonance elastography using a low-field dedicated system. J. Rheol. (N. Y. N. Y). 50, 531–541 (2006).
9. Vappou, J. et al. Magnetic resonance elastography compared with rotational rheometry for in vitro brain tissue viscoelasticity measurement. Magn. Reson. Mater. Physics, Biol. Med. 20, 273–278 (2007).
10. Vappou, J., Breton, E., Choquet, P., Willinger, R. & Constantinesco, A. Assessment of in vivo and post-mortem mechanical behavior of brain tissue using magnetic resonance elastography. J. Biomech. 41, 2954–2959 (2008).
11. Constantinesco, A. et al. Low-field dedicated and desktop magnetic resonance imaging systems for agricultural and food applications. Magn. Reson. Chem. 35, 69–75 (1997).
12. Yushchenko, M., Salameh, N. & Sarracanie, M. High-performance quadrature biplanar volume RF coil for flexible and fast in vivo imaging at low field (0.1 T). Book of Abstracts ESMRMB 2021 Online 38th Annual Scientific Meeting 7–9 October 2021. Magn. Reson. Mater. Physics, Biol. Med. 34, 162–163 (2021).
13. Yushchenko, M. et al. Elastography Validity Criteria Definition Using Numerical Simulations and MR Acquisitions on a Low-Cost Structured Phantom. Front. Phys. 9, (2021).
14. Bernstein, M. A. & Ikezaki, Y. Comparison of phase‐difference and complex‐difference processing in phase‐contrast MR angiography. J. Magn. Reson. Imaging 1, 725–729 (1991).
15. Muthupillai, R. et al. Magnetic resonance imaging of transverse acoustic strain waves. Magn. Reson. Med. 36, 266–274 (1996).
16. Debernard, L., Robert, L., Charleux, F. & Bensamoun, S. F. Characterization of muscle architecture in children and adults using magnetic resonance elastography and ultrasound techniques. J. Biomech. 44, 397–401 (2011).
17. Basford, J. R. et al. Evaluation of healthy and diseased muscle with magnetic resonance elastography. Arch. Phys. Med. Rehabil. 83, 1530–1536 (2002).
18. Bensamoun, S. F. et al. Determination of thigh muscle stiffness using magnetic resonance elastography. J. Magn. Reson. Imaging 23, 242–247 (2006).
19. Ito, D. et al. A new technique for MR elastography of the supraspinatus muscle: A gradient-echo type multi-echo sequence. Magn. Reson. Imaging 34, 1181–1188 (2016).
20. Numano, T. et al. A new technique for motion encoding gradient-less MR elastography of the psoas major muscle: A gradient-echo type multi-echo sequence. Magn. Reson. Imaging 63, 85–92 (2019).
21. Debernard, L., Leclerc, G. E., Robert, L., Charleux, F. & Bensamoun, S. F. IN VIVO CHARACTERIZATION OF THE MUSCLE VISCOELASTICITY IN PASSIVE AND ACTIVE CONDITIONS USING MULTIFREQUENCY MR ELASTOGRAPHY. J. Musculoskelet. Res. 16, 1350008 (2013).
22. Chakouch, M. K., Pouletaut, P., Charleux, F. & Bensamoun, S. F. Viscoelastic shear properties of in vivo thigh muscles measured by MR elastography. J. Magn. Reson. Imaging 43, 1423–1433 (2016).
23. Bilston, L. E. Soft tissue rheology and its implications for elastography: Challenges and opportunities. NMR Biomed. 31, 1–10 (2018).
Figures
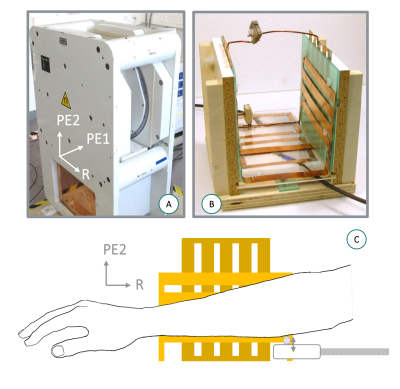
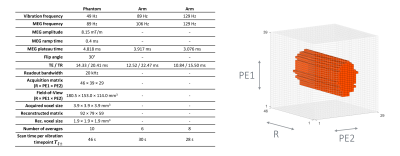
Figure 2: Left: MRE sequence parameters. Right: pattern of 3D k-space 10% sampling. R, PE1, PE2: readout, phase-encoding 1 and phase-encoding 2 directions.
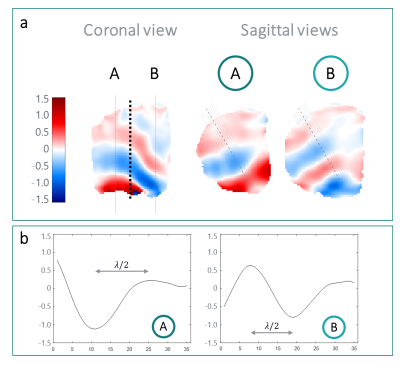
Figure 3. Phantom results with the 49-Hz vibration: a) phase maps of the encoded waves. Coronal view: the phantom compartments separation is indicated by a black dotted line; the dash-dot lines indicate the sagittal slices location in the stiffer (A) and in the softer (B) compartments. Sagittal view: the dotted segments represent the location of the extracted wave profiles. b) wave profiles along the middle segment (dashed line, sagittal views) in A and B.
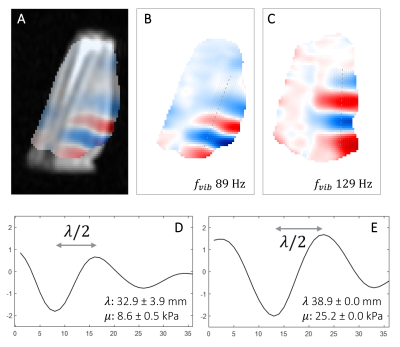
Figure 4: Phase-encoded wave information in the human forearm. A) overlay of phase map B with anatomy; B-C) phase maps acquired in two volunteers (sagittal), with segments indicating the profiles extracted for wavelength estimation, at 89 and 129 Hz vibration respectively; D-E) wave profiles along the middle segment, for B and C respectively.