0055
Importance of the lactate shuttle between astrocytes and neurons for brain activation1CRMSB, Bordeaux, France, 2Department of Physiology, Lausanne, Switzerland, 3I3M, Poitiers, France, 4Department of Biochemistry, Porto Alegre, Brazil, 5Laboratory of Cellular and Molecular Neurotherapies, Lausanne, Switzerland, 6Department of Pharmacology,, Porto Alegre, Brazil, 7IRTOMIT, Poitiers, France
Synopsis
For decades, it was claimed that glucose was the sole and sufficient energy substrate to sustain neuronal activity and brain function. Our results challenge this view by demonstrating that despite glucose availability, lactate shuttling from astrocytes to neurons via monocarboxylate transporters is necessary to give rise to the BOLD signal in the rat cerebral cortex following whisker stimulation. Moreover, lactate shuttling turned out to be also essential for sustaining behavioral performance associated with activation of the rat barrel cortex. These findings call for a reappraisal of neuroenergetics and the role of astrocytes in determining brain activation and function.
Introduction
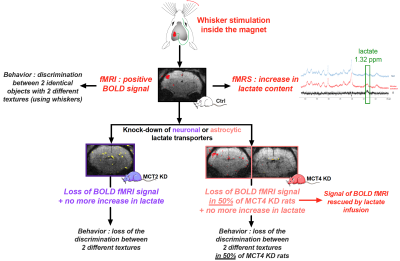
Glucose has always been considered the main substrate able to provide the energy necessary to sustain neuronal activity and brain function. However, in 1994, it was proposed that lactate, formed by astrocytes, can be shuttled to neurons, which can use it as an efficient energetic substrate1. Although numerous evidence have been provided over the last two decades that support the existence of such a mechanism, this shuttle has remained controversial2,3. A key step forward would be the in vivo demonstration that the transfer of lactate between astrocytes and neurons is essential to allow brain activation following a stimulation, and then to perform a cognitive task linked to the activation of the same specific brain region. In order to provide such a missing proof, we developed an adult rat model in which the expression of specific monocarboxylate transporters, responsible for lactate transport, has been down-regulated in a selective manner : either in astrocytes or neurons within the cortical brain region linked to whisker stimulation (the barrel cortex, or S1BF area)4. Our data indicate that lactate shuttling from astrocytes to neurons seems essential to obtain the characteristic signals associated with brain activation, as well as to accomplish a related specific behavioral task5.Materials and Methods
Experiments were conducted on a 7T Bruker BioSpec system equipped with a 20-cm horizontal bore. Animals: All experiments were conducted on three groups of Wistar rats 1) control, 2) genetically modified to down-regulate the neuronal lactate transporter MCT2 (MCT2 KD) and 3) genetically modified to down-regulate the astrocytic lactate transporter MCT4 (MCT4 KD). Rats were anaesthetized (for fMRS and fMRI experiments) using medetomidine hydrochloride (240µg/kg/h). Whisker stimulation was performed directly into the magnet using an air-puff system, which leads to the activation of a specific cortical brain area: the barrel cortex, or S1BF. fMRS: A voxel was located in the S1BF area (2x2.5x3mm) and in vivo spectroscopy was performed either at rest or during whisker activation using a LASER sequence (TE 20ms, TR 2500ms, 128 scans). Spectra were analyzed and lactate was quantified using LCModel and water-scaling. fMRI: The BOLD response was measured (using the activation paradigm) in four slices of 0.7 mm thickness using a single short gradient echo, echo planar imaging sequence (TR 500ms, TE 16.096ms, field of view 25x25mm², matrix size 96x96 and bandwidth of 33333Hz). Rescue experiments: Measurement of the BOLD response was performed twice. A first acquisition was performed during whisker activation, without lactate infusion. Then, lactate infusion (sodium salt, 534mM) was initiated and, 10min later, the second BOLD experiment was started. Lactate concentration was the highest in the barrel cortex within this time window, as previously determined by in vivo 1H-MRS performed every 5min in the barrel cortex during the lactate infusion protocol period (25min). Ex vivo MRS: Awake rats were infused with a solution containing [1-13C glucose]+lactate during whisker stimulations. S1BF areas (right -non activated- and left -activated-) were removed, metabolites (perchloric acid extraction) were analyzed on a Bruker DPX500 spectrometer equipped with a HRMAS probe using a POCE sequence to determine the 13C-specific enrichments. Behavioral studies: animals were submitted to 2 different novel object recognition (NOR) tasks: a “classical” one (vNOR), that tested the ability of the rats to discriminate between 2 different objects (using vision), and a “textured” one (tNOR), that tested the ability of the rats to discriminate between 2 similar objects but with 2 different textures (need the use of whiskers).Results
When either lactate transporters (MCT2 or MCT4) were downregulated in the rat barrel cortex, no lactate increase linked to whisker stimulation and measured by localized 1H-MRS in the S1BF could be observed, while this increase was detected in control rats. While a positive BOLD signal was observed in 95% of control rats, the BOLD signal was lost in MCT2 KD rats (only a small signal was observed in 4 out of 32 animals). In MCT4 KD, the BOLD signal was present in about half of the animals but absent in the other half. Rescue of the BOLD fMRI signal was obtained with lactate infusion in this non-responding MCT4 KD group. Concerning behavioral experiments, control rats were able to discriminate objects during both the vNOR and tNOR. MCT2 KD rats performed well in the vNOR task but they lost their ability to discriminate between 2 different textured objects in the tNOR task. Interestingly, as already observed during BOLD fMRI experiments, MCT4 KD rats can be divided into two sub-groups; a first sub-group, that was clearly unable to discriminate the textured objects (discrimination index, 0.49±0.04; 10/18 animals) while the second sub-group was able to discriminate the two different textured objects (discrimination index, 0.69±0.06; 8/18 MCT4-KD rats).Conclusion
Taken altogether our data indicate that lactate shuttling from astrocytes to neurons via monocarboxylate transporters is necessary to give rise to the BOLD signal in the rat cerebral cortex following whisker stimulation. Moreover, lactate shuttling turned out to be also essential for sustaining behavioral performance associated with activation of the rat barrel cortex. These findings call for a reappraisal of neuroenergetics and the role of astrocytes in determining brain activation and function.Acknowledgements
Anne-Karine Bouzier-Sore and Luc Pellerin have received financial support from an international French (ANR)/Swiss (FNS) grant, references ANR-15-CE37-0012 and FNS n°310030E-164271. Luc Pellerin also received financial support for this project from the program IdEx Bordeaux ANR-10-IDEX-03-02. Anne-Karine Bouzier-Sore has also received financial support from the French State in the context of the "Investments for the future" Programme IdEx and the LabEx TRAIL, reference ANR-10-IDEX and ANR-10-LABX-57, which also supported Helene Roumes. Nicole Déglon has received support from the BIOS and Panacée Foundations. Imad Benkhaled is supported by the LabCom I3M, Common Laboratory CNRS-Siemens, Univ. Poitiers and Poitiers University Hospital and by Region Nouvelle Aquitaine. Eduardo R. Zimmer is supported by CNPq [435642/2018-9] and [312410/2018- 2], Instituto Serrapilheira, reference Serra-1912-31365, Brazilian National Institute of Science and Technology in Excitotoxicity and Neuroprotection, reference 465671/2014-4, FAPERGS/MS/CNPq/SESRS–PPSUS, reference 30786.434.24734.23112017, ARD/FAPERGS, reference 54392.632.30451.05032021 and Alzheimer’s Association, reference AARGD-21-850670.References
1 Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A91, 10625-10629 (1994)
2 Bak, L. K. & Walls, A. B. CrossTalk opposing view: lack of evidence supporting an astrocyte-to-neuron lactate shuttle coupling neuronal activity to glucose utilisation in the brain. J Physiol596, 351-353, doi:10.1113/JP274945 (2018)
3 Barros, L. F. & Weber, B. CrossTalk proposal: an important astrocyte-to-neuron lactate shuttle couples neuronal activity to glucose utilisation in the brain. J Physiol596, 347-350, doi:10.1113/JP274944 (2018)
4 Jolle, C., Deglon, N., Pythoud, C., Bouzier-Sore, A. K. & Pellerin, L. Development of Efficient AAV2/DJ-Based Viral Vectors to Selectively Downregulate the Expression of Neuronal or Astrocytic Target Proteins in the Rat Central Nervous System. Front Mol Neurosci12, 201, doi:10.3389/fnmol.2019.00201 (2019)
5 Roumes, H.et al.Lactate transporters in the rat barrel cortex sustain whisker-dependent BOLD fMRI signal and behavioral performance. PNAS in press (2021).