0053
Toward a 1-minute high-resolution brain exam - MR Fingerprinting with fast reconstruction and ML-synthesized contrasts
Sophie Schauman1, Siddharth Srinivasan Iyer1,2, Mahmut Yurt1, Xiaozhi Cao1, Congyu Liao1, Zheng Zhong1, Guanhua Wang3, Greg Zaharchuk1, Shreyas Vasanawala1, and Kawin Setsompop1
1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Deptartment of Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States
1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Deptartment of Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States
Synopsis
MRI is a notoriously slow imaging method, but in recent years many technical developments have improved acquisition speed. However, many of these new fast sequences have not made their way to clinical practice. Barriers to uptake include long reconstruction times, diminished image quality, and non-standard contrast in the resulting images. Our objective is to translate a ~1 minute MRF sequence into clinical practice, providing five high quality images with common clinical contrasts at 1 mm isotropic-resolution, as well as quantitative T1, T2, and proton density maps, all within a 5 minute reconstruction pipeline that we have deployed clinically.
Introduction
Advances in magnetic resonance fingerprinting (MRF) acquisition and subspace-based reconstruction1,2,3 have enabled whole-brain quantitative, high-isotropic-resolution images to be obtained in minutes4. Concurrently, fast reconstruction5 and machine learning (ML) synthetized contrast-weighted images6,7 have also proven beneficial. In this work, we built upon these improvements, and combined tiny-golden-angle-shuffling multi-axis spiral projection (TGAS-SPI) MRF8, subspace reconstruction, and ML-based contrast-synthesis from subspace basis maps, to create a 1-minute quantitative and multi-contrast high-isotropic-resolution imaging scan with a fast and accurate reconstruction in < 5 minutes, deployed in a clinical setting. This provides exciting new opportunities for rapid clinical brain exams.Methods
The TGAS-SPI MRF sequence was acquired in 8 healthy adults and 7 pediatric patients on 3T GE Premier scanner (2:18min acquisition retrospectively subsampled to 1:09min). Additionally, for ML-training, the healthy volunteers underwent five clinical contrast-weighted scans at 1mm-isotropic resolution:- T1-Cube (5:05min. RPE/Rslice=2/1.5)
- T2-Cube (3:22min. RPE/Rslice=2/2)
- T2-FLAIR-Cube (6:21min. RPE/Rslice=2/2)
- T2-Double Inversion Recovery (WM Nulled)-Cube (7:53min. RPE/Rslice=2/2)
- T1-MPRAGE (4:57min. RPE/Rslice=2/1)
The subspace reconstruction was done on MRF data that were compressed from 500 to 20 frames using view-sharing, and coil-compressed to four channels. This memory reduction allowed a conjugate gradient iterative reconstruction to be deployed on a GPU for increased speed. The subspace basis maps from the MRF acquisition were then used to generate T1-, T2-, and PD-maps, using dictionary matching9, as well as synthesized conventional contrast images. The synthesized images were generated using a 2D generative adversarial network (GAN) applied to the basis maps (axial slices). Using basis maps as input retains temporal information related to e.g. partial volume and MT effects, which are not included in the parameter maps. The reconstruction pipelines are described in Figure 1a.
In previous work, a 1-minute TGAS-SPI MRF acquisition with subspace reconstruction and locally low-rank (LLR) constraint achieved good reconstruction, albeit with increased noise and minor spatial smoothing, and took ~4h to reconstruct10. With the proposed pipeline, no LLR regularization was applied, which results in an increase in noise in the basis maps. The GAN network therefore has the dual purposes of denoising and contrast-synthesis.
The synthesis network was based on the multi-task-deep-learning (MTDL) method11, originally developed for multi-contrast synthesis from the multi-echo MAGiC sequence12. The network input was the six phase aligned6 basis maps, and the output was one of the five contrasts listed above. Before training, the conventional contrast images were registered with the MRF data using FSL-FLIRT13. Six of the volunteers (910 slices) were used for training, one for validation, and one for testing. For the patients, only non-matched clinical scans acquired as part of the patients’ standard protocol were used for comparison. The ML-synthesis took 5s/contrast. A summary of the preprocessing and the GAN architecture used in this study are shown in Figure 1b-c.
In evaluating our proposed fast reconstruction, we compared the resulting synthetic images to ones from i) matched clinical sequences, ii) physics-based synthesis from the quantitative maps, and iii) ML-synthesized images from the basis maps of the previously demonstrated 4h LLR reconstruction.
Results
The synthesis network was able to recover all standard contrasts both from our proposed 5min reconstruction and the 4h reconstruction. The model based synthesis suffered from artifacts, especially in the T2-weighted images, as have been previously reported12 (Figure 2). This is likely explained by un-modelled MT effects that drive contrast in these images14. Figure 3 shows a zoomed-in example of a synthesized T2-FLAIR slice.Although the network was applied to axial slices, no strong striping artifact was observed in the coronal and sagittal planes, however in areas affected by non-rigid registration in training and B0-inhomogeneity, such as the eyes and neck, the network produced some blurring and textured artifacts (Figure 4).
The patient scans acquired on a different scanner, in a different age-group, and with pathology, were synthesized well despite these differences to the training data. One patient with a large post-surgical change and signal dropout due to a metal object is shown in Figure 5.
Discussion
We proposed and successfully evaluated an acquisition and reconstruction framework for quantitative and multi-contrast imaging with MRF that can be performed rapidly in the clinic with high quality and fast reconstruction. The pipeline is a concrete step toward moving fast MRI into clinical practice and providing both researchers and clinicians with multi-contrast qualitative and quantitative information.To further improve the method, incorporation of B0 and B1 maps that can be collected quickly with fast calibration scans, e.g. PhysiCal14 (=10s) would be beneficial. B0 incorporated into the recon will further reduce blurring in extreme B0 regions such as near the sinuses. B1 can be accounted for in dictionary fitting and to improve parameter mapping, particularly for T215. Another option to improve the parameter maps in 1 min acquisitions is to extend the ML approach for parameter mapping as well as contrast weighted synthesis.
Next steps include further network architecture optimization, obtaining a larger training dataset, and clinically validating the method (enabled by the fast acquisition and reconstruction pipeline.)
Acknowledgements
This study is supported in part by GE Healthcare research funds and NIH R01EB020613, R01MH116173, R01EB019437, U01EB025162, P41EB030006References
- Liang Z. Spatiotemporal imaging with partially separable functions. In: 2007 4th IEEE International Symposium on Biomedical Imaging: From Nano to Macro. Arlington, VA, USA: IEEE; 2007. pp. 988–991. doi: 10.1109/ISBI.2007.357020.
- Zhao B, Setsompop K, Adalsteinsson E, et al. Improved magnetic resonance fingerprinting reconstruction with low-rank and subspace modeling: A Subspace Approach to Improved MRF Reconstruction. Magn. Reson. Med. 2018;79:933–942 doi: 10.1002/mrm.26701.
- Assländer J, Cloos MA, Knoll F, Sodickson DK, Hennig J, Lattanzi R. Low rank alternating direction method of multipliers reconstruction for MR fingerprinting: Low Rank ADMM Reconstruction. Magn. Reson. Med 2018;79:83–96 doi: 10.1002/mrm.26639.
- Cao X, Liao C, Srinivasan S, et al. Optimized multi-axis spiral projection MRF with subspace reconstruction for rapid 1-mm isotropic whole-brain MRF in 2 minutes. In: Proc. Intl. Soc. Mag. Reson. Med. ; 2021.
- Gómez PA, Cencini M, Golbabaee M, et al. Rapid three-dimensional multiparametric MRI with quantitative transient-state imaging. Sci Rep 2020;10:13769 doi: 10.1038/s41598-020-70789-2.
- Wang K, Doneva M, Amthor T, et al. High Fidelity Direct-Contrast Synthesis from Magnetic Resonance Fingerprinting in Diagnostic Imaging. In: Proc. Intl. Soc. Mag. Reson. Med. ; 2020. p. 867.
- Qiu S, Chen Y, Ma S, et al. Direct Synthesis of Multi-Contrast Images from MR Multitasking Spatial Factors Using Deep Learning. In: Proc. Intl. Soc. Mag. Reson. Med. 29. ; 2021. p. 2429.
- Cao X, Ye H, Liao C, Li Q, He H, Zhong J. Fast 3D brain MR fingerprinting based on multi‐axis spiral projection trajectory. Magn Reson Med 2019;82:289–301 doi: 10.1002/mrm.27726.
- Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013;495:187–192 doi: 10.1038/nature11971.
- Cao X, Liao C, Iyer SS, et al. Optimized multi-axis spiral projection MR fingerprinting with subspace reconstruction for rapid whole-brain high-isotropic-resolution quantitative imaging. arXiv:2108.05985 [physics] 2021.
- Wang G, Gong E, Banerjee S, et al. Synthesize High-Quality Multi-Contrast Magnetic Resonance Imaging From Multi-Echo Acquisition Using Multi-Task Deep Generative Model. IEEE Trans. Med. Imaging 2020;39:3089–3099 doi: 10.1109/TMI.2020.2987026.
- Tanenbaum LN, Tsiouris AJ, Johnson AN, et al. Synthetic MRI for Clinical Neuroimaging: Results of the Magnetic Resonance Image Compilation (MAGiC) Prospective, Multicenter, Multireader Trial. AJNR Am J Neuroradiol 2017;38:1103–1110 doi: 10.3174/ajnr.A5227.
- Jenkinson M, Bannister P, Brady M, Smith S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 2002;17:825–841 doi: 10.1006/nimg.2002.1132.
- Iyer SS, Liao C, Li Q, et al. PhysiCal: A rapid calibration scan for B0, B1+, coil sensitivity and Eddy current mapping. In: Proc. Intl. Soc. Mag. Reson. Med. ; 2020. p. 661
- Conklin J, Cli B, Feiweier T, et al. A comprehensive multi-shot EPI protocol for high-quality clinical brain imaging in 3 minutes. In: Proc. Intl. Soc. Mag. Reson. Med. 28. ; 2020. p. 300.
Figures
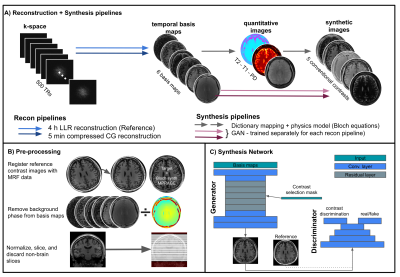
Figure 1
A) Pipelines used for reconstruction and synthesis. The proposed pipeline is the dark blue + pink arrows, the others pipelines were set up and tested as reference.
B) The pre-processing steps performed on the data from healthy volunteers. For patients the registration step was omitted because no matched reference was acquired.
C) is a sketch of the network used for synthesis (based on Wang et al.11)
A) Pipelines used for reconstruction and synthesis. The proposed pipeline is the dark blue + pink arrows, the others pipelines were set up and tested as reference.
B) The pre-processing steps performed on the data from healthy volunteers. For patients the registration step was omitted because no matched reference was acquired.
C) is a sketch of the network used for synthesis (based on Wang et al.11)
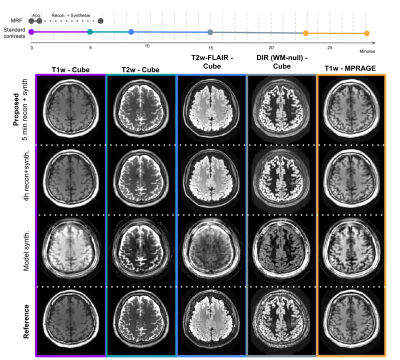
Figure 2
Top: Timeline comparing the MRF acquisition+reconstruction (approx. 6 minutes, of which the patient is only required to be still for 1 minute) with the time it took to acquire the conventional contrasts (approx. 28 minutes).
Below: Five synthesized contrasts in the test subject using the proposed method (row 1), a much slower reconstruction (row 2), a simple model based synthesis (row 3), and the reference scans (row 4).
Top: Timeline comparing the MRF acquisition+reconstruction (approx. 6 minutes, of which the patient is only required to be still for 1 minute) with the time it took to acquire the conventional contrasts (approx. 28 minutes).
Below: Five synthesized contrasts in the test subject using the proposed method (row 1), a much slower reconstruction (row 2), a simple model based synthesis (row 3), and the reference scans (row 4).
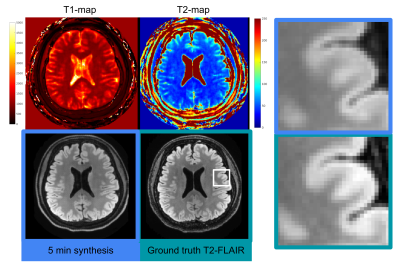
Figure 3
Left: Example showing high quality synthesis of T2-FLAIR (bottom left) despite a somewhat noisy T2 map (top right). The T1 map (top left) is cleaner, so it is possible the network learned to use information arising both from T1 and T2 decay to create the synthetic images. On the reference T2 FLAIR image (bottom right) the white box indicates the area of the zoomed-in inserts.
Right: Zoomed in T2-FLAIR images (top: 5 min synthesis, bottom: reference) showing clear delineation of the cortex and gray and white matter boundary.
Left: Example showing high quality synthesis of T2-FLAIR (bottom left) despite a somewhat noisy T2 map (top right). The T1 map (top left) is cleaner, so it is possible the network learned to use information arising both from T1 and T2 decay to create the synthetic images. On the reference T2 FLAIR image (bottom right) the white box indicates the area of the zoomed-in inserts.
Right: Zoomed in T2-FLAIR images (top: 5 min synthesis, bottom: reference) showing clear delineation of the cortex and gray and white matter boundary.
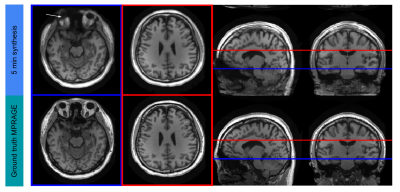
Figure 4
Example of MPRAGE synthesis showing two slices, one with some artifact (white arrow) and one clean image. In the coronal and sagittal views one can see that the network is consistent and not producing striping artifacts despite being a 2D network acting on each axial slice individually.
Example of MPRAGE synthesis showing two slices, one with some artifact (white arrow) and one clean image. In the coronal and sagittal views one can see that the network is consistent and not producing striping artifacts despite being a 2D network acting on each axial slice individually.
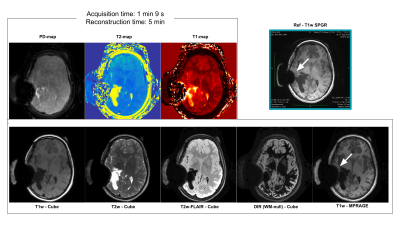
Figure 5
Five clinical contrast scans along with three quantitative maps for a patient with a large post surgical change and dropout artifact (white arrows). These 8 images are available from only a 1:09 min scan and 5 mins of reconstruction and synthesis. Note that the dropout artifact is of similar severity as the spoiled gradient echo (top right) scan that was acquired as part of the patient’s standard care. Note also that the network that generated the five contrasts in the bottom row had never encountered images containing these features in its training, suggestive of its robustness.
Five clinical contrast scans along with three quantitative maps for a patient with a large post surgical change and dropout artifact (white arrows). These 8 images are available from only a 1:09 min scan and 5 mins of reconstruction and synthesis. Note that the dropout artifact is of similar severity as the spoiled gradient echo (top right) scan that was acquired as part of the patient’s standard care. Note also that the network that generated the five contrasts in the bottom row had never encountered images containing these features in its training, suggestive of its robustness.
DOI: https://doi.org/10.58530/2022/0053