0052
Automated Sequence Design using Neural Architecture Search1Department of Electrical and Computer Engineering, Seoul National University, Seoul, Korea, Republic of, 2Division of Biomedical Engineering, Hankuk University of Foreign Studies, Gyeonggi-do, Korea, Republic of
Synopsis
A new pulse sequence design method that requires no prior knowledge of MR physics or training dataset is proposed. This method utilizes neural architecture search, generating an optimal sequence for given properties (e.g., T2*, B1+) and target objectives. It successfully discovered FLAIR-like and spin-echo-like sequences. Furthermore, a less-intuitive sequence using three RF pulses was created when targeted for spin-echo. In an in-vivo experiment, this new sequence had almost identical contrasts and SNR with spin-echo (our SNR: 297.1±70.1; spin-echo SNR: 295.8±78.2) while utilizing 90% of RF energy of the spin-echo, suggesting a potential of the automated sequence design.
Introduction
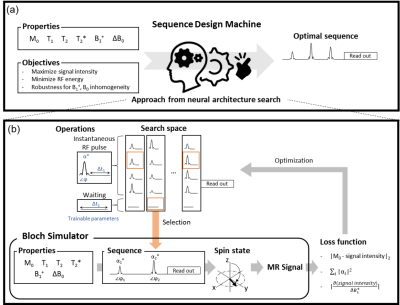
Developing a sequence requires extensive knowledge of MR physics and, therefore, currently available sequences are limited by human intuition due to non-linearity in the Bloch equations and spin dynamics. Recently, AI-powered design methods have been proposed in various fields.1-3 Among them, automated sequence design methods have produced GRE-like sequences for target objects4-6 and performed optimization in existing sequences4. In this study, a new sequence design method is proposed using neural architecture search (NAS) to create pulse sequences without any prior knowledge of sequences or MR physics and without preparation of training dataset. In the current version, we confined our task to designing RF pulse parameters (e.g., number of pulses, flip angles, phases, delay between the pulses or readout).Methods
Our method is designed to produce an optimal sequence for given tissue properties (M0, T1, T2, T2*), imaging properties (B1+, ΔB0), and target objectives. Hence, these properties are inputted to the Bloch simulator to generate MR signals. Using the signals, a loss function defined by the objective is calculated for optimization. Then the optimization process determines the sequence design (e.g., number of pulses, flip angles (α), phase (φ), duration after RF pulse (Δt1) or waiting duration (Δt2)) using NAS (Fig. 1). NAS was utilized to optimize a series of operations (i.e., sequence) by replacing a neural architecture and its weight parameters with a sequence design and its scan parameters, respectively. An example is given in Figure 1b where RF pulses and waiting duration are determined from search space and concatenated to generate a sequence. ProxylessNAS7 was utilized, which optimizes architecture parameters with back-propagation of the loss function.[Simulation] Bloch simulator was implemented to consider relaxations, T2* effect, and magnetic field inhomogeneities. The simulator generated signals for a voxel. The T1 and T2 relaxation effects were applied during the idle and waiting durations. For the T2* effect, a number of isochromats were created, dephasing in a Lorentzian distribution. The B1+ factor was multiplied to the flip angle of the RF pulses. The B0 perturbation induced the phase shift of each isochromat.
[Experiments] To test our method, two experiments were performed to design a FLAIR-like sequence and a spin-echo-like sequence (Fig. 2).
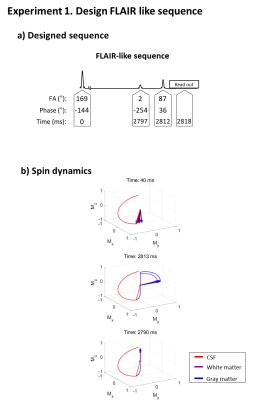
Experiment 1: Null signal from cerebrospinal fluid (CSF) and maximize signals from white matter and gray matter.
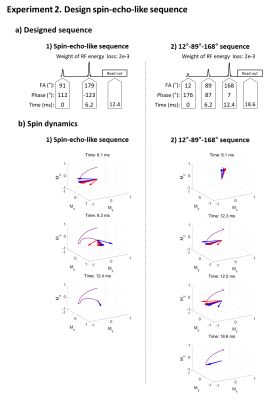
Experiment 2: Maximize signal intensity while minimizing dependency of B1+ and B0 perturbation.
For both experiments, ranges of tissue properties were assumed as summarized in Figure 2 (M0 was 1).
The loss function contained signal intensity loss and RF energy loss. The signal intensity loss was defined as L2 loss between signal intensity and M0 for signal maximization, and between signal intensity and zero for nulling. The loss function for RF energy was designed to create a low RF energy sequence. The dependency of the perturbation was formulated as a derivative of signal intensity with respect to B1+ or B0 at B1+ = 1.0 and ΔB0 = 0 Hz. The repetition of the sequence was neglected assuming TR to be infinity. The maximum number of RF pulses was confined to 5. The minimum idle time between RFs or between RF and readout was set to 6.2 ms. RF pulses with less than 1° were discarded. In each experiment, a sequence was created four times and the best performing one was chosen as the final sequence.
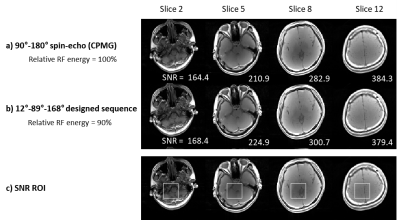
The final sequences were compared to conventional sequences (Experiment 1 for FLAIR; Experiment 2 for CMPG spin-echo). In Experiment 2, in-vivo images using the final sequence and the spin-echo sequence were acquired from one subject: TR = 1,000 ms, FOV = 224 × 224 mm2, in-plane resolution = 1.8 × 1.8 mm2, slice thickness = 5 mm, and the number of slices = 16. The signal-to-noise ratio (SNR) of a region of interest (ROI) inside the brain and RF energy were compared.
Results
In Experiment 1, a FLAIR-like sequence was successfully generated (Fig. 3), revealing an inversion-like pulse at the beginning of the sequence then a long weighting duration for gray/white matter signal recovery and CSF signal suppression, which was followed by an excitation-like pulse. An unexpected 2° pulse existed between the two RF pulses but had little or no effect. The spin dynamics were also similar to FLAIR.In Experiment 2, our method created two results: a spin-echo-like sequence and a less intuitive three RF pulse (12°-89°-168°) sequence behaving like spin-echo (Fig. 4; see spin dynamics). As shown in Figure 5, this new three-RF sequence produced identical contrasts and similar SNR with the spin-echo images (new sequence SNR: 297.1±70.1 vs. spin-echo SNR: 295.8±78.2). The new sequence had reduced RF energy (90% of the spin-echo), while taking a longer duration (18.6 ms vs. 12.4 ms).
Discussion and Conclusion
In this work, a novel automatic sequence design method requiring no prior knowledge or training data was proposed. The method automatically created sequences from the properties and target objectives. The results showed successful designs of FLAIR-like and spin-echo-like sequences. In addition, a less-intuitive spin-echo-like sequence using three RF pulses was generated with reduced RF energy. This study suggests a potential for automated sequence design that may explore possibilities beyond human intuition.Acknowledgements
This work was supported by Creative-Pioneering Researchers Program through Seoul National University (SNU) and the National Research Foundation of Korea (NRF-2021R1A2B5B03002783).References
[1] Shin, D., Kim, Y., Oh, C., An, H., Park, J., Kim, J., & Lee, J. (2021). Deep Reinforcement Learning Designed RadioFrequency Waveform in MRI. Nature Machine Intelligence, accepted.
[2] Sanchez-Lengeling, B., & Aspuru-Guzik, A. (2018). Inverse molecular design using machine learning: Generative models for matter engineering. Science, 361(6400), 360–365.
[3] Mirhoseini, A., Goldie, A., Yazgan, M., Jiang, J. W., Songhori, E., Wang, S., … & Dean, J. (2021). A graph placement methodology for fast chip design. Nature, 594(7862), 207–212.
[4] Loktyushin, A., Herz, K., Dang, N., Glang, F., Deshmane, A., Weinmüller, S., ... & Zaiss, M. (2021). MRzero‐Automated discovery of MRI sequences using supervised learning. Magnetic Resonance in Medicine, 86(2), 709-724.
[5] Zhu, B., Liu, J., Koonjoo, N., Rosen, B., & Rosen, M. (2018). AUTOmated pulse SEQuence generation (AUTOSEQ) using Bayesian reinforcement learning in an MRI physics simulation environment. In Proc. 26th Annu. Meeting ISMRM.
[6] Samuel, S. W. (2019). Using deep reinforcement learning to actively adaptively and autonomously control of a simulated MRI scanner. In Proc. 27th Annu. Meeting ISMRM
[7] Cai, H., Zhu, L., & Han, S. (2019). ProxylessNAS: Direct neural architecture search on target task and hardware. 7th International Conference on Learning Representations, ICLR 2019, 1–13.
Figures