0043
Diffusion-Weighted Imaging at 0.064 T1Hyperfine, Inc, Guilford, CT, United States, 2Neurology, Massachusetts General Hospital, Boston, MA, United States, 3Yale New Haven Hospital, New Haven, CT, United States
Synopsis
A sequence is described for DWI on a portable, 0.064T scanner. Images in a healthy subject and a patient with pathology are shown.
Introduction
Conventional MRI is impractical in many settings because of its cost, siting needs, weight, and power consumption. This motivates the development of low cost, portable, MRI systems [1, 2, 3]. Design constraints limit such systems to low-field, making SNR a key consideration. While lower SNR makes diffusion-weighted imaging (DWI) a challenge, DWI at field strengths as low as 0.064T has been used to successfully image stroke [4, 5]. Here we describe a DWI sequence and associated artifact mitigation strategies that are practical for use in a portable, low-field, MRI system. We show image quality obtainable in a laboratory environment in a healthy volunteer as well as a case with clinically relevant pathology obtained in a hospital setting at the point-of-care.Methods
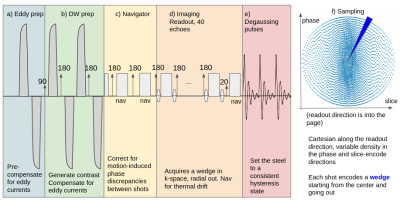
MRI: Data were acquired on a 0.064 T portable system (Swoop, Hyperfine Inc, Guilford, USA) with max gradient 24mT/m. The diffusion-weighted sequence was a dual spin echo 3D fast spin echo sequence (Figure 1). Parameters were: TR=1s, TE=90ms, echo spacing 4.8ms, echo train length of 40, b=90 0s/mm2, 22 x 18 x 20 cm, resolution = 2.4x 2.4 x 6mm, scan time=8.5 min, with diffusion encoding direction along the patient’s A/P axis. Encoding was Cartesian along the readout and a pseudo random sampling in the phase-slice directions, and oversampled by 5X to get sufficient SNR. Due to a large amount of steel the system has many uncompensated eddy currents, so an eddy current preparation (Figure 1a) was added to set the system in a steady state prior to the DWI prep. Diffusion encoding was performed with shaped bipolar pulses (Figure 1b). The first two echoes were used as navigators to correct for rigid body motion (Figure 1c). After the echo train (Figure 1d) a low tip angle free induction decay (FID) was collected to correct for thermal drift in the magnet blocks, followed by a degaussing block that resets the hysteresis state of the system (Figure 1e). In addition to the eddy current precompensation a hysteresis correction is performed [6]. Low b-value images (b=10s/mm2) are acquired at a scan time of 2 min and used to compute apparent diffusion coefficient (ADC) maps.Reconstruction: Image data were denoised with sensor based denoising [7]. Next the navigator echoes are used to phase correct each echo train [8] separately for even and odd echoes using the respective navigators. The even and odd echoes are treated separately in the system due to uncorrected eddy currents that cause differential warping between the two. Even and odd echoes are separately reconstructed with an iterative SENSE reconstruction [9] and then registered using advanced normalization tools library [10]. Images were also denoised using deep convolutional neural network based denoising (DL) [11].
Human subjects: A healthy volunteer (M/39 y.o.) was scanned under IRB. A second subject (M/68 y.o.) presented comatose to the Massachusetts General Hospital (MGH) emergency department with a CT diagnosis of right thalamic hemorrhage extending into ventricles.
Results
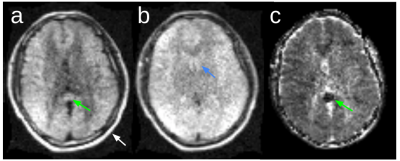
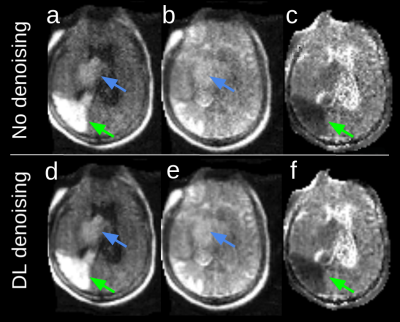
Healthy volunteer: Figure 2 shows image quality in a healthy volunteer without DL denoising. Due to the single readout direction the white matter shows evidence of anisotropic signal as expected in the high-b-value image (Figure 2a). The low-b-value image (Figure 2b) has T2 weighting, but with lower CSF signal than expected due to short T2 (1s).Patient with pathology: Imaging was completed at the bedside following admission from the ED. The DWI scan shows a clinically unexpected acute ischemic infarct (Figure 3, green arrows) in the right temporo-parietal region of the brain, in addition to the right thalamic intraparenchymal hemorrhage (Figure 3, blue arrows). The contrast in the lesion is notably higher than any observable white matter anisotropy effects demonstrating that the anisotropy in the white matter does not hinder visualization of the ischemic pathology. Hypointensity on ADC (Figure 3c, green arrow) confirms restricted diffusion in the ischemic lesion. DL-based denoising (Figure 3 d,e,f) improves the appearance of the images while maintaining the depiction of the lesions.
Discussion and Conclusion
There are some differences in the appearance of these images to a conventional, high field DWI. In the interest of time, a single encoding direction was used. One limitation of this approach is the detection of normal white matter anisotropy. For the application of stroke it can be a challenge to interpret, although as it was in the case shown, stroke pathology has a higher contrast than white matter anisotropy. Fat-suppression is not used due to the lack of EPI ghosting. Also due to the lack of EPI readout and low field there is no T2* contrast in the b=0 images. This must be noted to radiologists interpreting the images so that they do not assume they will be able to see bleeds on the low-b-value images.In conclusion we demonstrated the features of a DWI sequence implemented on a portable, low-field MRI system. While the resolution and SNR are lower than a conventional system, this sequence was able to detect clinically relevant pathology. In settings where conventional imaging is unavailable, impractical, or simply not feasible in a timely manner, this sequence may provide clinicians with a valuable diagnostic tool.
Acknowledgements
No acknowledgement found.References
[1] Sheth KN, Mazurek MH ,Yuen MM, Cahn BA, Shah JT, Ward A, Kim JA, Gilmore EJ, Falcone GJ, Petersen N, Gobeske KT, Kaddouh F, Hwang DY, Schindler J, Sansing L, Matouk C, Rothberg J, Sze G, Siner J, Rosen MS, Spudich S, Kimberly WT. Assessment of BrainInjury Using Portable, Low-Field Magnetic Resonance Imaging at the Bedside of Critically Ill Patients. JAMA Neurol. 2020 Sep 8 :e203263.doi:10.1001/jamaneurol.2020.3263. Epub ahead of print.PMID:32897296;PMCID:
[2] Wald LL, McDaniel PC, Witzel T, Stockmann JP, Cooley CZ. Low- cost and portable MRI. J Magn Reson Imaging. 2020;52:686- 696.
[3] Marques JP, Simonis FFJ, Webb AG. Low- field MRI: an MR phys-ics perspective. J Magn Reson Imaging. 2019;49:1528- 1542
[4] O’Halloran R, Sacolick L, Dyvorne H, Lazarus C, By S, Welch EB, Cahn BA, Shah J, Mazerek M, Yuen MM, Sze G, Kimberly T, Rosen M, Sheth K. Stroke Imaging at 64mT .Proceedings of the 28th Annual Meeting of the International Society for Magnetic Resonance in Medicine. 2020.
[5] Yuen M, Mazurek M, Cahn B, Prabhat A, By S, Houchun H, Welch EB, Sacolick L, O’Halloran R, Ward A, Timano N, Falcone G, Gilmore E, Hwang D, Kim J, Kaddouh, F, Sharma R, Amin H, Schindler J, Matouk C, Herbert R, Wira CR, Sze G, Rosen M, Kimberly WT, Sheth K. Qualitative Description of Ischemic Stroke Appearance on Low-Field, Point-Of-Care Magnetic Resonance Imaging. International Stroke Conference 2021 Oral Abstracts #33.
[6] O’Halloran R, Hugon C, Sacolick L, Dyvorne H. Correcting for hysteresis in magnetic resonance imaging. US Patent Application US20200209334A1. November 15, 2019
[7] Rearick T, Charvat G, Rosen MS, Rothberg J. Noise Suppression Methods and Apparatus. US Patent 9,797,971. March 10, 2016
[8] Ordidge R, Helpern J, Qing Z, Knight R, Nagesh V. Correction of motionalartifacts in diffusion-weighted MR images using navigator echoes. MagnReson Imaging 1994;12:455–460
[9] Pruessmann, K. P., Weiger, M., Börnert, P., & Boesiger, P. (2001). Advances in sensitivity encoding with arbitrary k‐space trajectories. Magnetic Resonance in Medicine, 46(4), 638-651.
[10] Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009 Jul 1;46(3):786-802. doi: 10.1016/j.neuroimage.2008.12.037. Epub 2009 Jan 13. PMID: 19195496; PMCID: PMC2747506.
[11] Schlemper J, Dey N, Salehi SSM, Lazarus C, O’Halloran R, Kundu P, Sofka M. Unsupervised Denoising for Low-field Diffusion MRI. Proceedings of the 2021 ISMRM & SMRT Annual Meeting & Exhibition. Abstract Number 2190.
Figures