0042
High-fidelity submillimeter-isotropic-resolution diffusion MRI through gSlider-BUDA and circular EPI with S-LORAKS reconstruction1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 3Radiologic Technology Department, Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand, 4Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 5Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 6Department of Radiology, Harvard Medical School, Boston, MA, United States, 7Stanford Center for Cognitive and Neurobiological Imaging, Stanford University, Stanford, CA, United States
Synopsis
BUDA-cEPI combines a circular-EPI (cEPI) trajectory with Blip-Up and Down Acquisition (BUDA), to achieve high-fidelity diffusion imaging. It employs full-ramp-sampling to efficiently cover a circular k-space to shorten the readout-train and mitigate T2*-blurring. For further readout-shortening, a combined phase-encoding(PE) & readout(RO) partial-Fourier (pF) is employed, where PE-pF is resolved via opposing the blip-up and down EPI-shots, and S-LORAKS reconstruction is used to effectively fill-out the remaining k-space. This resulted in an acquisition with markedly-reduced TE and ~40% reduced echo-train-length and T2*-blurring compared to standard-EPI at the same Rinplane and PE-pF. To achieve high-SNR, BUDA-cEPI is also combined with a gSlider-multi-slab acquisition.
Introduction
Submillimeter-isotropic-resolution dMRI has shown great potential for characterizing white-/gray-matter(1). The key challenges of submillimeter in-vivo dMRI are the decreased SNR, physiological-noise-induced phase variations, and image distortions from B0-inhomogeneity and eddy-current. To address these issues, our recent work(2,3) proposed the BUDA-EPI framework that employs multi-shot interleaved blip-up and -down phase-encodings and reconstruction which incorporates B0 forward-modeling and structured-low-rank constraint to enable distortionless and navigator-free dMRI. Combined with the SNR-efficient gSlider technique(4), it enables high-fidelity diffusion imaging across the whole-brain at sub-millimeter-resolution. However, the original BUDA uses standard-EPI trajectory, which at high resolution can result in lengthy echo-train length (ETL) and undesirable T2*-induced image-blurring.In this work, we combine circular-EPI(5–7) with PE- and RO-pF sampling, to reduce the ETL and image-blurring. With BUDA, the variable ESP of circular-EPI are incorporated into the model-based reconstruction to correct image-distortions. By enforcing the acquired two-shot data into the structured-low-rank reconstruction, the phase variations across the two-shot are mitigated, providing high-fidelity diffusion-weighted images without need of navigators. Using the proposed technique, we demonstrate high-fidelity 0.72- and 0.50-mm-isotropic-resolution dMRI with TE of 55ms and 66ms, respectively, and ~40% reduction of ETL.
Methods
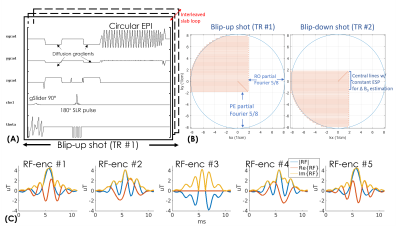
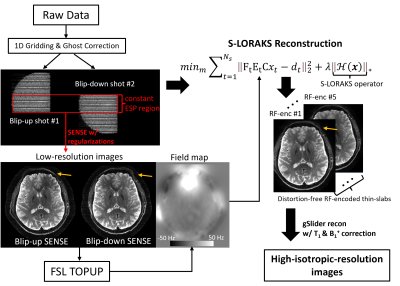
Pulse sequence: Figure1(A) shows the sequence-diagram of gSlider-BUDA-circular-EPI(Fig.1(B))., where two EPI-shots sample complementary k-space to create opposing distortion. As shown in Fig.1(B), circular-EPI only acquires ~30% k-space area, using both RO- and PE-pF and full ramp-sampling, which significantly reduce ETL and TE. The central part of k-space is acquired using a constant echo-spacing to enable individually-reconstructed blip-up and -down low-resolution images from this central region to be used for off-resonance estimation. Five gSlider RF-slab-encodings (Fig.1(C)) are used for a total of 10 EPI-shots per volume across blip-up and -down-encodings, where both shots are acquired in an interleaved fashion. The sequence programming uses KSFoundation(https://ksfoundationepic.org/).Reconstruction: The reconstruction of each gSlider-encoding (Fig2) includes: (i) Nyquist ghost correction with a constant gradient-delay and linear phase-error estimated from a calibration scan with Gy turned off. Due to the variable ESP and ramp-sampling in circular-EPI, 1D NUFFT is performed ky-line-by-ky-line. (ii) The central k-space of each shot is reconstructed using SENSE with L1-wavelet regularization. The reconstructed low-resolution images of a BUDA pair are used to estimate field-map via FSL TOPUP(8). (iii) This field map is then incorporated into a model-based joint-reconstruction with a structured-low-rank constraint (S-LORAKS(9,10)) across the two shots to take advantage of the smooth background phase for pF-filling and account for shot-to-shot phase variations.
The proposed reconstruction is performed for each RF-encoding volume, and subsequently, the processed RF slab-encoded volumes are combined in a forward-model-based reconstruction with T1/B1+ corrections to create a high slice-resolution volume as per(11).
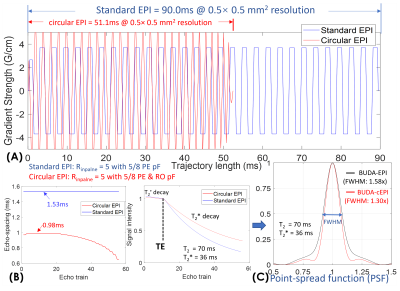
Simulations: To validate the advantages of circular- over standard-EPI, we simulated both circular- and standard-EPI trajectory at 0.50mm-resolution with in-plane acceleration 5 with 5/8 PE-pF for both circular- and standard-EPI, and 5/8 RO-pF for circular-EPI only. The maximum gradient and slew-rate of circular-EPI were set to 50mT/m and 150T/m/s, while the standard-EPI trajectory is directly extracted from a product sequence with maximum gradient and slew rate of 37mT/m and 188T/m/s, with 30% ramp-sampling. The point-spread-functions (PSF) were calculated for both circular- and standard-EPI to characterize T2* decay-induced blurring.
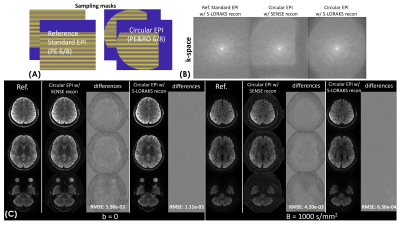
To characterize the performance of S-LORAKS in reconstructing missing pF data of BUDA-cEPI, BUDA-cEPI with 6/8 RO- & PE-pF was simulated from BUDA-standard-EPI with 6/8 PE-pF dataset using a circular sampling mask(Fig.4(A)) and the resulting reconstruction compared to that of BUDA-standard-EPI with no RO-pF.
Acquisitions: In-vivo brain-data were acquired with gSlider-BUDA-cEPI at 0.72- and 0.50-mm-isotropic-resolution on a 3T GE UHP scanner with a NOVA 32-channel head-coil. A low-resolution B1+ map was obtained to correct the B1+-inhomogeneity-induced slab-profile imperfection in gSlider-reconstruction using a 29-second Bloch-Siegert sequence.
Results
Figure3 shows the comparison between circular- and standard-EPI at 0.50mm resolution. The trajectory lengths of circular- vs standard-EPI are 51.1ms vs 90.0ms (Fig.3(A)), which demonstrate a ~40% reduction in ETL (Fig.3(B)). The minimum TEs of circular- vs standard-EPI are 66ms vs 73ms. The signal curves with T2’- and T2*-decay during echo-trains were simulated in Fig.3(B), and the corresponding PSFs were calculated with different reconstructions shown in Fig.3(C). With S-LORAKS reconstruction, the PSF of BUDA-standard-EPI is expected to be 1.58x that of the actual resolution, while the PSF of BUDA-cEPI is 1.30x, resulting in a significant reduction in image-blurring for 0.50 mm EPI-data.Figure4 shows the reconstruction performance comparison of BUDA-standard-EPI vs. synthesized BUDA-cEPI with additional RO-pF generated from the same dataset. Figure4(B) demonstrates that BUDA-cEPI reconstructed using SENSE without S-LORAKS resulted in missing high k-space data, while incorporating S-LORAKS into the reconstruction can help effectively recover the missing k-space, leading to small root-mean-square-error(RMSE) in both b=0 & 1000 s/mm2 images (Fig.4(C)).
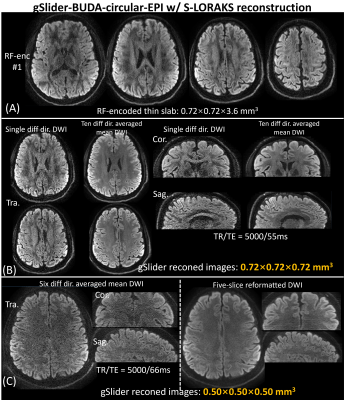
Figure5(A) shows the reconstructed RF-encoded thin-slabs, and (B) shows representative diffusion-weighted and 10 diffusion-directions averaged slices at 0.72-mm-isotropic-resolution. Figure 5(C) shows the 6 diffusion-directions averaged slices at 0.50-mm-isotropic resolution and five-slice reformatted DWI in three orthogonal views.
Discussion and conclusion
In this work, we developed a gSlider-BUDA-circular-EPI with readout and phase-encoding pF acquisition, together with model-based S-LORAKS reconstruction to achieve high-fidelity dMRI at sub-millimeter resolution. In comparison to standard-EPI, the proposed method provides improved-SNR and reduced blurring.Acknowledgements
This study is supported in part by GE Healthcare research funds and NIH R01EB020613, R01MH116173, R01EB019437, U01EB025162 , P41EB030006.References
1. Leuze CWU, Anwander A, Bazin PL, et al. Layer-specific intracortical connectivity revealed with diffusion MRI. Cereb. Cortex 2014;24:328–339 doi: 10.1093/cercor/bhs311.
2. Liao C, Bilgic B, Tian Q, et al. Distortion-free, high-isotropic-resolution diffusion MRI with gSlider BUDA-EPI and multicoil dynamic B0 shimming. Magn. Reson. Med. 2021;86:791–803 doi: 10.1002/mrm.28748.
3. Liao C, Cao X, Cho J, Zhang Z, Setsompop K, Bilgic B. Highly efficient MRI through multi-shot echo planar imaging. In: Lu YM, Papadakis M, Van De Ville D, editors. Wavelets and Sparsity XVIII. Vol. 11138. SPIE; 2019. p. 43. doi: 10.1117/12.2527183.
4. Setsompop K, Fan Q, Stockmann J, et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magn. Reson. Med. 2018;79:141–151 doi: 10.1002/mrm.26653.
5. Rettenmeier C, Maziero D, Qian Y, Stenger VA. A circular echo planar sequence for fast volumetric fMRI. Magn. Reson. Med. 2019;81:1685–1698 doi: 10.1002/mrm.27522.
6. Kerr AB, Pauly JM, Hu BS, et al. Real-time interactive MRI on a conventional scanner. Magn. Reson. Med. 1997;38:355–367 doi: 10.1002/mrm.1910380303.
7. Pauly J, Butts K, Pat GL, Macovski A. A circular echo-planar pulse sequence. In: Proc., SMR, Third Scientific Meeting. Nice, France; 1995. p. 106.
8. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. Fsl. Neuroimage 2012;62:782–790 doi: 10.1016/j.neuroimage.2011.09.015.
9. Haldar JP. Low-Rank Modeling of Local k-Space Neighborhoods (LORAKS) for Constrained MRI. IEEE Trans. Med. Imaging 2014;33:668–681 doi: 10.1109/TMI.2013.2293974.
10. Kim TH, Setsompop K, Haldar JP. LORAKS makes better SENSE: Phase-constrained partial fourier SENSE reconstruction without phase calibration. Magn. Reson. Med. 2017;77:1021–1035 doi: 10.1002/mrm.26182.
11. Liao C, Stockmann J, Tian Q, et al. High-fidelity, high-isotropic-resolution diffusion imaging through gSlider acquisition with B1+ and T1 corrections and integrated ΔB0/Rx shim array. Magn. Reson. Med. 2020;83:56–67 doi: 10.1002/mrm.27899.
Figures