0032
Spatial Scaling of Respiratory-Triggered Liver Diffusion Weighted Imaging1Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Department of Physics, Technical University of Munich, Garching, Germany, 3Philips Healthcare, Eindhoven, Netherlands, 4Philips GmbH Market DACH, Hamburg, Germany
Synopsis
Physiological motion induces signal loss in diffusion-weighted imaging of the liver even when respiratory triggering is employed. Cardiac motion mostly affects the left liver lobe resulting in an inhomogeneous signal appearance and overestimation of the apparent diffusion coefficient (ADC). Spatial scaling is an algorithm that handles the intrinsic weighting of signal by rejecting bad acquisitions and voxels with subsequent calculation of a spatially dependent scaling factor for the final averaging. The present work shows that by adding such a spatial scaling postprocessing step, liver signal homogeneity can be increased, and overestimation of the ADC can be reduced.
Purpose
Diffusion weighted imaging (DWI) is considered an asset in clinical detection of liver lesions and a tool for assessing liver fibrosis 1-5. Diffusion encoding sensitizes motion of molecules within tissue, making it prone to motion artifacts caused by cardiac, respiratory, or bulk motion in body DWI 1,4,6,7. The left liver lobe is particularly affected by internal motion due to its proximity to heart and lungs resulting in signal loss in DWI and overestimation of the apparent diffusion coefficient (ADC) 1,6,7. Therefore, liver appears inhomogeneous and pathologies in the left lobe may be harder to detect, even when a respiratory-triggered DWI acquisition is employed 2,7,8. Since multiple averages are obtained per b-value and direction, the final image results from averaging individual acquisitions 9. The present work showcases a novel algorithm that intervenes prior to the averaging and aims to reduce the artificial signal loss in respiratory-triggered DWI by spatially-scaled averaging.Methods
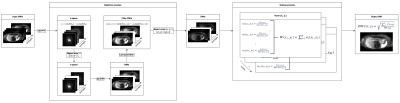
Fig. 1 depicts the whole workflow of the proposed algorithm. Its underlaying assumption is that data is naturally weighted by signal loss which is not considered in the averaging process. Therefore, we introduced the new scaling factor in the divisor of the averaging equation.Algorithm
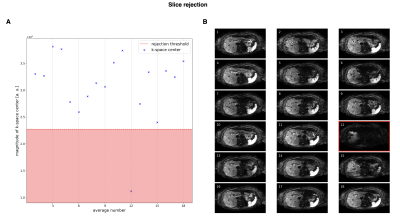
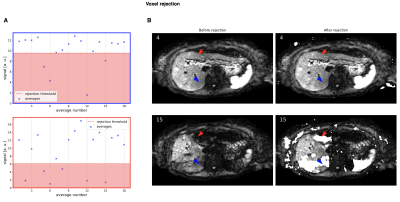
Outlier rejection is a two-step process with slice rejection and voxel rejection performed successively. For slice rejection, the $$$N$$$ averages are transformed into k-space to compare their center frequencies $$$K_0=\{k_1(0,0),...,k_N(0,0)\}$$$ and reject those below the threshold $$$t_1$$$ with the median of k-space centers, their median absolute deviation (MAD), and parameter $$$\lambda_1>0$$$. The voxel threshold is defined by the median of magnitudes of a single voxel $$$I(x,y)=\{I_1(x,y),...,I_N(x,y)\}$$$, their MAD, and parameter $$$\lambda_2>0$$$. Both parameters are set to a high value of 3 to only reject slices and voxels with severe signal dropout.
The last step is data scaling with weights computed on the rejected data. For their computation, the signal is normalized by the maximum signal for each voxel and the corresponding individual weights are summed up to $$$W(x,y)=\sum_{i=1}^N{w_i(x,y)}$$$. This factor is used to scale the resulting DWI which can be used for diagnosis or to compute other diffusion parameters like ADC.
MR measurements
72 abdominal patients were scanned on a $$$3\,\mathrm{T}$$$ whole-body scanner (Ingenia Elition, Philips Healthcare) using the 16-channel torso coil and the built-in-table 12-channel posterior coil. A spin echo DW sequence in combination with a single-shot EPI readout was employed with the following acquisition parameters: $$$FOV=420\mathrm{x}370\,\mathrm{mm}^2$$$; voxel size $$$=3\mathrm{x}3\mathrm{x}4\,\mathrm{mm}^3$$$; $$$60$$$ slices with $$$0.4\,\mathrm{mm}$$$ gap; b-values $$$=0,50,300,600\,\mathrm{s}/\mathrm{mm}^2$$$ with $$$4,1,2,6$$$ averages; and 3 diffusion encoding directions ($$$b>0$$$). All scans were respiratory triggered with a fixed trigger delay of $$$200\,\mathrm{ms}$$$. Reconstruction was performed off-line using standard and spatially-scaled averaging. ADC estimation was performed using the averaged DWIs with $$$b>0$$$. The b600 DWIs from the standard and proposed spatially-scaled averaging methods were blindly evaluated by a radiologist regarding image quality, liver homogeneity, perceived signal-to-noise ratio (SNR), and quality of lesion detection.
Results
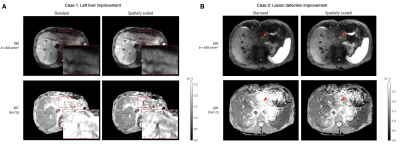
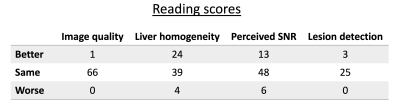
k-space center magnitudes of a single slice and their corresponding averages are depicted in Fig. 2. The plots in Fig. 3A represent the signal magnitude of all averages across one slice of two specific voxels indicated in Fig. 3B. The resulting images in Fig. 4 exhibit a higher left liver lobe signal and increased homogeneity of the whole liver with the spatial scaling method applied compared to the standard averaging. The overshoot in the left liver in the ADC image is diminished when using the proposed method, hence the bias in ADC has been reduced.Out of 72 data set, four were not diagnostic and one only partly covered the liver resulting in 67 data sets for evaluation.A summary of the radiological reading is listed in Tab. 1. There was a trend for higher liver homogeneity with the spatial scaling method (mean score $$$3.13\pm0.92$$$) compared to the standard method (mean score $$$2.82\pm0.89$$$) with statistical significance of $$$p=0.05$$$, but without any statistical differences between the two methods regarding image quality $$$(p=0.93)$$$, perceived SNR $$$(p=0.51)$$$, and lesion detection $$$(p=0.53)$$$.
Discussion and Conclusion
The proposed spatial scaling algorithm is a data-driven approach to mitigate the effect of signal loss in the left liver lobe that results from internal motion and to achieve a better estimate of ADC. DWIs as well as ADC maps display a more homogeneous liver in the case of signal loss, possibly aiding the detection of lesions. In case of predominant or even total signal loss, the signal cannot be recovered, and the image quality cannot be increased. A concern to the resulting image is the apparent drop in SNR of a few, especially originally bad images, although the results of the radiological reading were not significantly different to the standard method.In conclusion, liver DWI can be improved in terms of homogeneity and the recovery of signal in the left lobe using the proposed spatial scaling method. ADC maps display accordingly more homogeneous structure and less overestimation in the left liver. A slight increase in quality of detection of lesions was noted for some cases but must be further evaluated. Rejection threshold parameters should be set so that only motion affected averages and voxels are rejected and may be changed to be adaptive to the data in future work.
Acknowledgements
The present work was supported by Philips Healthcare.References
1. Kele PG, van der Jagt EJ. Diffusion weighted imaging in the liver. World J Gastroenterol. 2010;16(13):1567-1576
2. Nasu K, Kuroki Y, Fujii H, Minami M. Hepatic pseudo-anisotropy: a specific artifact in hepatic diffusion-weighted images obtained with respiratory triggering. MAGMA. 2007;20(4):205-211
3. Nasu K, Kuroki Y, Seikiguchi R, Nawano S. The Effect of Simultaneous Use of Respiratory Triggering in Diffusion-weighted Imaging oft the Liver. Magn Reson Med Sci. 2006;5(3):129-136
4. Saito K, Tajima Y, Harada TL. Diffusion-weighted imaging of the liver: Current applications. World J Radiol. 2016;8(11):857-867
5. Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254(1):47-66
6. Koh DM, Scurr, E, Collins DJ, Pirgon A, Kanber B, Karanjia N, Brown G, Leach MO, Husband JE. Colorectal hepatic metastases : quantitative measurements using single-shot echo-planar diffusion-weighted MR imaging. Eur Radiol. 2006;16:1898-1905
7. Kwee TC, Takahara T, Niwa T, Ivancevic MK, Herigault G, van Cauteren M, Luijten PR. Influence of cardiac motion on diffusion-weighted magnetic resonance imaging of the liver. Journal of Magnetic Resonance Imaging. 2009;35(2):318-327
8. Nasu K, Kuroki Y, Nawano S, Kuroki S, Tsukamoto T, Yamamoto S, Motoori K, Ueda T. Hepatic Metastases: Diffusion-weighted Sensitivity-encoding versus SPIO-enhanced MR Imaging. Radiology. 2006;239(1)
9. Merboldt KD, Bruhn H, Frahm J, Gyngell ML, Hänicke W, Deimling M. MRI of “Diffusion” in the Human Brain: New Results Using a Modified CE-FAST Sequence. Magnetic Resonance in Medicine. 1989;9(3):423-429
Figures