0029
Free-breathing liver fat quantification with combined multi-echo Dixon and 3D radial stack-of-stars in children and adults at 3T and 1.5T1Philips GmbH Market DACH, Hamburg, Germany, 2Diagnostic and Interventional Radiology, University Hospital RWTH Aachen, Aachen, Germany, 3Philips Japan, Tokyo, Japan, 4Department of Diagnostic and Interventional Radiology and Nuclear Medicine, Section of Pediatric Radiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Synopsis
Multi-echo gradient-echo Dixon based on the chemical shift method is currently the standard for non-invasive in vivo quantification of the liver fat but requires breath-holding. In young children or sick patients this is often challenging or not feasible. Despite recent attempts using different free-breathing approaches to mitigate patient motions, these are not widely available or routinely used. In this work we employ the multi-echo Dixon sequence with 3D pseudo-golden angle radial stack-of-stars readout for free-breathing liver fat assessment and report our initial results in children and adults at 3T and 1.5T with phantom validation and comparison to the standard method.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disorder and has high prevalence in the western world in both adults and children 1,2. Multi-echo gradient-echo Dixon MRI based on the chemical shift method and Cartesian trajectory is considered the non-invasive reference standard for spatially resolved quantification of liver proton-density fat fraction (PDFF) 3-4. However, its examination requires breath-holding and is often difficult or not feasible in young children and patients with severe illness or old age. Recent attempts combining non-Cartesian trajectories such as 3D radial stack-of-stars have shown promising results mitigating respiratory motions 5-7. However, their use remains limited due to the need of extra calibrations, offline reconstruction and long scan time. In this work we aimed to (i) employ multi-echo Dixon and 3D pseudo-golden angle radial stack-of-stars for liver fat assessment in free breathing, (ii) investigate its robustness in image quality and PDFF assessment using phantom validation and in vivo measurements in children and adults at both 3T and 1.5T, with comparison to the reference standard.METHODS
Imaging pulse sequence: For free-breathing liver fat quantification, the multi-echo radiofrequency (RF) spoiled gradient-echo Dixon sequence was combined with a 3D radial stack-of-stars k-space trajectory in a pseudo-golden angle sampling order 8. The latter has a more homogeneous distribution of the consecutively acquired radial spokes in space and time comparing to standard golden-angle, which leads to a lower correlation of variations in terms of phase errors caused by motion or hardware imperfections such as trajectory delay 8,9. A radial density of 145% referring to the Nyquist theorem for a corresponding image matrix size in the XY-plane was applied, while a SENSE (SENSitivity Encoding) factor of 1.2 was used in the Z-direction for scan time reduction.Phantom validation: A commercially available phantom (Fat Fraction Phantom Model 300, Calimetrix, Madison, WI, USA) was used. It consists of a spherical acrylic housing containing twelve fat fraction compartments submerged in a doped water bath. Standard multi-echo Dixon sequences based on standard Cartesian and free-breathing radial stack-of-stars trajectories were performed. Detailed imaging parameters are summarized in Table 1. All images including proton density fat fraction (PDFF) maps were reconstructed on the scanner host PC by the vendor software (MR Release R5.6 and R5.7, Philips Healthcare, Best, The Netherlands). Complex signal fitting using a 7-peak fat model and T2* correction was performed for PDFF calculation as previously described 10,11.
Patient exams: All patients with previously diagnosed or suspected fatty liver disease (FLD) underwent MRI at 3.0T or 1.5T (Ingenia and Ingenia Ambition, Philips Healthcare, Best, The Netherlands) with 70 cm bore and a standard 32-channel torso coil. Both the standard breath-holding Cartesian and the free-breathing pseudo-golden angle radial stack-of-stars were included in the imaging protocol and applied in a random order. The latter was performed without respiratory triggering or navigator gating. Imaging parameters were the same as described above in the phantom measurement.
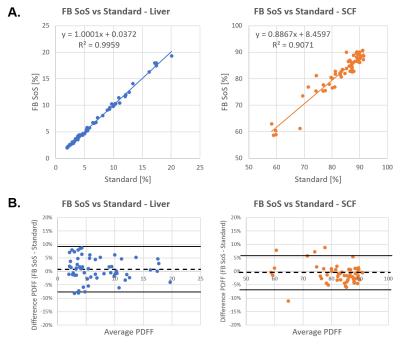
For analysis source images and hepatic proton-density fat fraction (PDFF) maps were compared by visual inspection for image quality. Region of interests (ROIs) were drawn on the PDFF maps in the liver as well as subcutaneous fat (SCF) without inclusion of image artifacts. Free-breathing and standard PDFF values were compared using linear correlation and Bland-Altman analysis 12. P<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
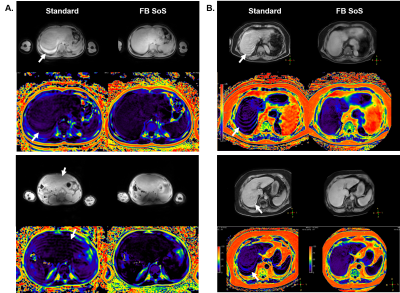
Fat fraction phantom results demonstrate excellent agreement between the pseudo-golden angle radial stack-of-stars and the standard (r=0.996, P<0.05), as seen in Figure 1. For in vivo exams, in total 59 patients were studied (aged 51 ± 25 years [mean ± SD], range 1 to 83 years, 25 females). 3D free-breathing stack-of-stars clearly demonstrated less motion artifacts compared to the Cartesian standard acquired in breath-hold. Representative cases are shown in Figure 2. Free-breathing PDFF demonstrated a linear relationship versus standard Cartesian PDFF (r=0.998, P<0.01). The Bland-Altman plots indicated very small mean differences in PDFF between FB stack-of-stars versus standard Cartesian (average difference <1% for both liver and subcutaneous fat), as shown in Figure 3.CONCLUSION
This work investigated 3D pseudo-golden angle radial stack-of-stars for liver fat quantification acquired in free breathing at 3T and 1.5T. Initial results in children and adults demonstrate improved image quality with less respiratory motion artifacts and excellent comparability to the PDFF assessed by the conventional Cartesian method as the current standard reference. These results justify a more widespread clinical investigation of the method to improve patient compliance in both pediatric and adult patients and facilitate the clinical adoption for quantification of liver fat.Acknowledgements
The authors thank Rieke Lisa Meister for her assistance in preparing the clinical imaging protocol and Robert Lay for useful discussion.
References
1. Marjot T, Moolla A, Cobbold JF, et al. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocrine Reviews 2020;41:bnz009.
2. Schwimmer JB, Deutsch R, Kahen T, et al Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388.
3. Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, quantitative assessment of liver fat by MRI‐PDFF as an endpoint in NASH trials. Hepatology 2018;68:763.
4. Kühn JP, Hernando D, Muñoz del Rio A, et al. Effect of multipeak spectral modeling of fat for liver iron and fat quantification: correlation of biopsy with MR imaging results. Radiology 2012;265:133.
5. Armstrong T, Martin T, Stemmer A, et al. Free-breathing fat quantification in the liver using a multiecho 3D stack-of-radial technique: investigation of motion compensation and quantification accuracy. Proc 25th Annual Meeting ISMRM, Honolulu, USA; 2017. p 363.
6. Armstrong T, Dregely I, Stemmer A, et al. Free-breathing liver fat quantification using a multiecho 3D stack-of-radial technique. Magn Reson Med 2018;79:370.
7. Armstrong T, Ly KV, Murthy S, et al. Freebreathing quantification of hepatic fat in healthy children and children with nonalcoholic fatty liver disease using a multi-echo 3-D stack-of-radial MRI technique. Pediatr Radiol 2018;48:941.
8. Hedderich DM, Weiss K, Spiro JE, et al. Clinical Evaluation of Free-Breathing Contrast-Enhanced T1w MRI of the Liver using Pseudo Golden Angle Radial k-Space Sampling. Fortschr Röntgenstr 2018;190:601.
9. Winkelmann S, et al. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging 2007;26:68.
10. Meisamy S, Hines CD, Hamilton G, et al. Quantification of hepatic steatosis with T1-independent, T2*-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 2011;258:767.
11. Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am 2010;18:337.
12. Bland JM and Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;327:307.Figures
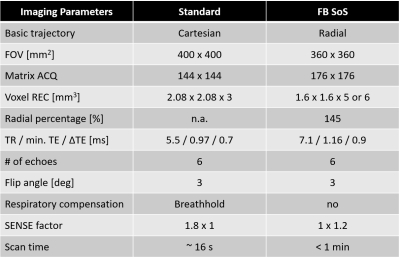
Table1: Imaging pulse sequences used for liver fat quantification in this study.
FB SoS = free-breathing stack-of-stars; FOV = field of view; ACQ = acquired; REC = reconstructed; TR = repetition time; TE = echo time; SENSE = sensitivity encoding
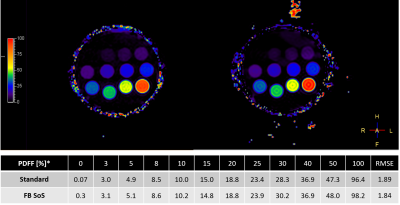
Figure 1: Fat fraction phantom measurement using both standard method and free-breathing radial stack-of-stars (FB SoS) sequence. The measured PDFF mean values of the phantom vials are displayed in the table below the images. Scale on the left indicates fat fraction in %. RMSE = root mean square error.
*Phantom vials spec with 1.5% tolerance of the absolute proton density fat fraction (PDFF)