0028
Integrated high-order B0 shimming for multiparametric quantitative liver imagingat 3T using a UNIfied Coil (UNIC)1Stanford University, Stanford, CA, United States, 2Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Siemens Medical Solutions, Los Angeles, CA, United States
Synopsis
Liver MRI shows promise for rich morphologic and physiologic information. B0 inhomogeneity causes off-resonance spread at the liver-lung interfaces, degrading image quality and quantification accuracy for DWI, T1, and T2*/R2*. A novel high-order shim coil (UNIC) was built, minimizing the decoupling between the two overlapped shim and RF arrays, allowing the free design of shim loop topology in proximity of the target organ. Improved shimming from UNIC is demonstrated with increased FOV in liver imaging, showing significantly reduced distortion in liver DWI, increased image quality score of MOLLI T1 map, and reduced B0-offset-induced ADC, R2*, and B0 standard deviations.
Introduction
MRI plays an essential role in liver imaging, providing superior contrast resolution and rich morphologic and physiologic information1. T1 mapping can indicate fibrosis severity2; R2*/T2* is correlated with iron concentration3; diffusion-weighted echo-planar-imaging (DWI-EPI) offers information about liver masses4. However, B0 inhomogeneity, especially at high fields (>=3T), may significantly degrade the image quality, compromise the reliability of the underlying MRI contrast and thus limit more widespread clinical application of quantitative measurements such as ADC and R2*/T2*5. B0 inhomogeneity in the liver at 3T (particularly at the liver-lung interfaces) can lead to a 200Hz off-resonance spread, causing distortion on DWI-EPI, banding artifacts on T1 mapping with bSSFP, and reduced accuracy in T2*/R2* quantification5. Recently, a new high-order shim coil by integrating a local shim array into an RF coil, named UNIfied Coil (UNIC), was developed by our group6-8. UNIC minimizes the decoupling between the two closely overlapped shim and RF arrays, allowing the free design of shim loop topology in proximity of the target organ for maximizing shim efficacy. Also, shim field strength can be multiplied in favor of shimming internal organs by enabling multiple-turn shim loops compared to the iPRES ‘AC/DC’ coils9-11. Previous studies showed UNIC’s capability of improving B0 homogeneity in the myocardium6-8. In this work, UNIC's performance in liver imaging was tested with increased FOV. The hypothesis was that improved shimming from UNIC can alleviate the distortion of DWI-EPI, eliminate banding artifacts on bSSFP, and increase the precision of T2*/R2* quantification of the liver.Methods
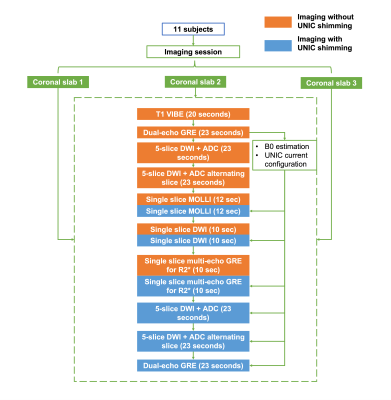
Shimming coil: The in vivo studies were approved by the local IRB and written informed consent was obtained from all participants. Eleven healthy volunteers were scanned on a 3T Siemens Biograph mMR scanner. The UNIC coil prototype for B0 shimming and RF reception was used7-8 which demonstrated uncompromised SNR by adding the UNIC shim array to the RF coil.Imaging experiments: All the images were acquired with breath-holds and in coronal orientation for the visualization of B0-inhomogeneity-induced image degradation at the liver lung interface. In each imaging session, two to three coronal slabs with a thickness of 40mm each were acquired for whole-liver coverage. For each slab, MRI images and field maps were acquired under scanner shim and UNIC shim. Experiments were conducted sequentially. Images were first acquired under mGRE-based scanner shim using the scanner-equipped 2nd order spherical harmonic shimming (without UNIC shim current) and next under UNIC shim applied to the identical shim volume.
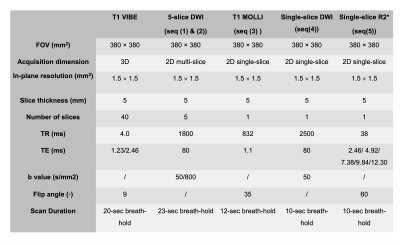
Image acquisition: A 3D fat-suppressed T1-VIBE (GRE-based) sequence with whole-liver coverage was performed for fine localization of liver segments and reference for distortion-free image. A dual-echo GRE sequence was performed next covering the target slab for B0 calculation and current configuration for UNIC shimming. The following set of sequences were performed twice without or with UNIC current: (1) 2D 5-slice DWI-EPI sequence with two b-values (50/800s/mm2), slice thickness=5mm, slice gap=5mm, breath-hold=23s; (2) 2D 5-slice DWI-EPI at alternating slices to fill up the slice gap in (1); (3) 2D single-slice modified look-locker (MOLLI) using bSSFP at the slab center with slice thickness=5mm, breath-hold=12s; (4) 2D single-slice DWI-EPI at slab center with one b-value=50s/mm2, slice thickness=5mm, breath-hold=10s; (5) 2D single-slice multi-echo GRE at center slice for the estimation of R2* with slice thickness=5mm, breath-hold=10s. For sequence #4 and #5, acquisitions without and with UNIC shimming occurred in the same 20s breath-hold to minimize location mismatch. The imaging workflow and parameters are demonstrated in Figure 1 and Table 1. After the acquisitions, a dual-echo GRE scan was performed to assess the B0 field with UNIC shimming.
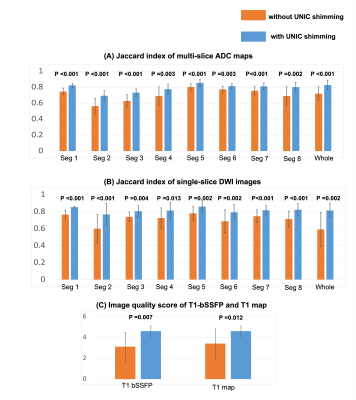
Image analysis: The liver was divided into 8 segments according to Couinaud criteria. The distortion was compared with T1-VIBE and measured by Jaccard index for the ADC maps from sequence #1 and the DWI from sequence #4. The Jaccard indices for each segment and the entire liver were assessed and compared between image sets without and with UNIC. MOLLI T1 mapping quality was reviewed and a score ranging from 1 (lowest) to 5 (highest) was given to each set. The standard deviations of the ADC, R2*, and B0 for each segment and the entire liver were evaluated.
Results
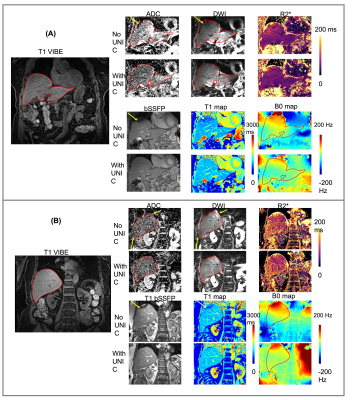
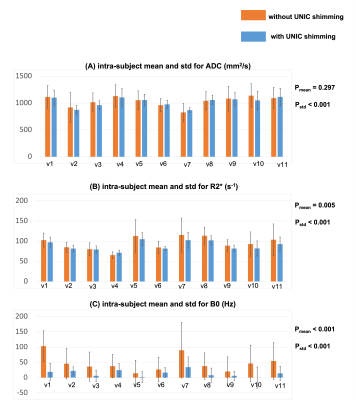
Figure 2 compares images without and with UNIC for an anterior slice (A) and a posterior slice (B). With UNIC shim, distortions on DWI and ADC images were visually reduced, banding artifacts on bSSFP and T1 from MOLLI were removed, and B0 was more homogeneous. Figure 3 displays the quantitative comparison without and with UNIC on morphology. For ADC maps and DWI, the mean Jaccard index for each segment and the entire liver among all volunteers were significantly improved with UNIC. The image quality score of MOLLI was also significantly increased. Figure 4 illustrates the spatial distribution of ADC, R2*, and B0 values for each subject. With UNIC shim, the intra-subject standard deviations among different segments of the liver for ADC, R2*, and B0 were significantly reduced and B0 offset was significantly decreased.Discussions and Conclusion
The study demonstrates the potential of UNIC coil shimming in reducing B0 inhomogeneity and consequently improving the distortion of DWI-EPI, removing banding artifacts of bSSFP, and increasing ADC and R2* quantification precision of the whole liver.Acknowledgements
Authors extend their gratitude to Drs. Bernd Stoeckel, Fraser Robb, and Miguel Navarro. Drs. Hsin-Jung Yang, Debiao Li, and Hui Han contributed equally to this work. This work was supported by NIH R01 HL156818; R01 NS121544; U01 EB028145.References
1. Vu LN., Morelli JN, and Szklaruk J. "Basic MRI for the liver oncologists and surgeons." Journal of hepatocellular carcinoma 5 (2017): 37.
2. Banerjee R, Pavlides M, Tunnicliffe EM, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. Jan 2014;60(1):69-77.
3. Hernando D, Levin YS, Sirlin CB, Reeder SB. Quantification of liver iron with MRI: state of the art and remaining challenges. J Magn Reson Imaging. Nov 2014;40(5):1003-21.
4. Shenoy-Bhangle A, Baliyan V, Kordbacheh H, Guimaraes AR, and Kambadakone A. "Diffusion weighted magnetic resonance imaging of liver: Principles, clinical applications and recent updates." World journal of hepatology 9, no. 26 (2017): 1081.
5. Roberts NT, Hinshaw LA, Colgan TJ, Ii T, Hernando D, and Reeder SB. "B0 and B1 inhomogeneities in the liver at 1.5 T and 3.0 T." Magnetic Resonance in Medicine 85, no. 4 (2021): 2212-2220.
6. Yang H, Stager J, Azab L, Liu W, Lu M, Huang Y, et al. Whole Heart High-Order B0 Shimming at 3T Using a UNIfied Coil (UNIC) for RF Receive and Shimming. Proceedings of the 28th Scientific Annual Meeting of the ISMRM Virtual Meeting. 2020(Abstract 2183).
7. Yang H, Liu W, Stager J, Xie YB, Selvin S, Azab L, et al. Overcoming off-resonance limitations in high field CMR with high-order local whole heart B0 shimming using a UNIfied shim-RF Coil (UNIC). The Society for Cardiovascular Magnetic Resonance (SCMR) 22nd Annual Scientific Sessions Young Investigator Award finalist Highlighted at Siemens user's meeting. 2019.
8. Han H. Multi-Coil B0 Shimming in the Body. In: Proceedings of the 28th Annual Meeting of ISMRM, Virtual Meeting. 2020:E0912.
9. Han H, Song AW, Truong TK. Integrated parallel reception, excitation, and shimming (iPRES). Magnetic resonance in medicine. 2013;70(1):241-7.
10. Truong TK, Darnell D, Song AW. Integrated RF/shim coil array for parallel reception and localized B0 shimming in the human brain. Neuroimage. 2014;103:235-40.
11. Stockmann JP, Witzel T, Keil B, Polimeni JR, Mareyam A, LaPierre C, et al. A 32-channel combined RF and B0 shim array for 3T brain imaging. Magn Reson Med. 2016;75(1):441-51.
Figures