0021
Free-breathing High-resolution Spiral Real-time Cardiac Cine Imaging at 1.5 T with DEep learning-based Spiral Image REconstruction (DESIRE)1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2School of Artificial Intelligence, Beijing University of Posts and Telecommunications, Beijing, China, 3Medicine, University of Virginia, Charlottesville, VA, United States, 4Radiology, University of Virginia, Charlottesville, VA, United States, 5Medicine, Stanford University, Stanford, CA, United States
Synopsis
Cardiac magnetic resonance (CMR) real-time cine, which does not require breath-holding or ECG gating, is clinically useful particularly for patients with impaired breath-hold capacity and/or arrhythmias. Spiral acquisitions, which provide high acquisition efficiency and insensitivity to motion artifacts, can require a long reconstruction time particularly for compressed-sensing or other iterative reconstruction techniques. As such they cannot provide immediate feedback to the imager. Here, we sought to develop high-resolution real-time cine imaging at 1.5 T using fast spiral acquisitions and deep learning-based rapid imaging reconstruction for both bSSFP and GRE imaging, to make high-quality and online reconstruction for cine imaging feasible.
Introduction
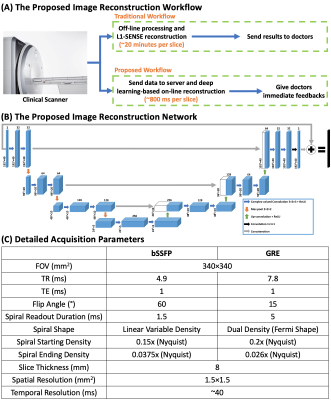
Cardiac magnetic resonance (CMR) real-time cine imaging, which doesn’t require ECG gating and breath-holding, is more clinically valuable than breath-hold cine MRI for patients with impaired breath-hold capacity and/or arrhythmias1–7. Currently, clinically available cardiac real-time cine imaging techniques with Cartesian acquisition using parallel imaging might suffer from limited spatial-temporal resolution, while techniques using compressed sensing require long reconstruction times hence they cannot provide immediate feedback to doctors. Spiral imaging techniques, which have high acquisition efficiency, enable cine acquisition with high spatial-temporal resolution. However, iterative compressed sensing-based image reconstruction of spiral imaging is time-consuming. Here, we aim to develop high-resolution real-time cardiac cine imaging at 1.5 T using rapid spiral acquisitions and deep learning-based imaging reconstruction for both bSSFP and GRE imaging, to make high-quality and rapid online reconstruction for cardiac cine imaging feasible (Figure 1-A).Methods
Data Acquisition and Image Reconstruction for Reference Images 2D bSSFP and GRE cine image series with 1.5×1.5 mm2 in-plane spatial resolution and whole-heart coverage were acquired from 9 patients and 9 healthy volunteers undergoing clinical studies (Figure1-C) on a 1.5 T SIEMENS scanner (MAGNETOM Aera; Siemens Healthineers, Erlangen, Germany)8. The data was acquired using a 16-second free-breathing continuous acquisition where spiral interleaves were rotated by the golden angle between subsequent TRs. Every 5 spirals were combined into a frame which led to a temporal resolution of around 40 ms.The reference image series for the network training was generated using the non-Cartesian spiral L1-SENSE reconstruction9,10 which can be formulated as:
$$\underset{x}{\operatorname{argmin}}\left\|F_{u} S x-y\right\|_{2}^{2}+\lambda\|\Psi x\|_{1}$$
where $$$x$$$ is the dynamic image series to be reconstructed, $$$S$$$ is the coil sensitivity maps estimated from the temporal average image using a method described by Walsh et al11, $$$F_u$$$ is the inverse Fourier gridding operator that transforms the Cartesian image space to the spiral k-space12, $$$y$$$ is the acquired spiral k-space data, $$$\Psi$$$ is the finite time difference sparsifying operator, and $$$\lambda$$$ balances between data consistency and sparsity. $$$\lambda=0.06M_0$$$ was chosen as a tradeoff between image quality and temporal fidelity, where $$$M_0$$$ was the maximal magnitude value of the NUFFT image series. The objective function was solved using non-linear conjugate gradient algorithm with 30 iterations.
Prior to the image reconstruction, coil selection8 was conducted on the NUFFT-gridded12 multi-coil image series at each slice location to reduce spiral aliasing artifacts.
Proposed Image Reconstruction Network Figure 1-B shows the proposed 3D U-Net13 based image reconstruction network. The inputs to the network were single-channel complex-valued under-sampled dynamic image series after NUFFT gridding and optimal coil combination14. The real and imaginary parts of the data were concatenated into two channels and the complex-valued convolution operation was enforced15. The outputs were concatenated real and imaginary dynamic image series reconstructed using spiral L1-SENSE served as reference images.
Experimental Setup To save the GPU memory, each image series was cropped into a 192×192 matrix with 40 temporal frames, corresponding to 1.6 seconds of cine data. This would be sufficient to capture a single beat for a heart rate above 40 beats per minute. Each dynamic image series signal was normalized to 0-1. The training of the network was conducted using PyTorch on four NVIDIA Tesla P100 GPUs (12 GB memory each) for 150 epochs with a batch size of 4 and an L1 loss (absolute error) function using an ADAM optimizer.
120 slices from 13 subjects were used for training, and another 14 slices from 5 subjects were used for testing. bSSFP and GRE image reconstruction networks were trained separately.
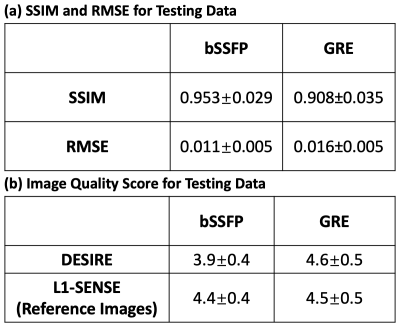
Image Analysis bSSFP and GRE testing data were reconstructed using both DESIRE and spiral L1-SENSE. Both structural similarity index (SSIM)16 and root mean square error (RMSE) for the images reconstructed using DESIRE were assessed with respect to the reference images (spiral L1-SENSE).
Reconstructed image series were also blindly graded by an experienced cardiologist (5, excellent; 1, poor).
Results
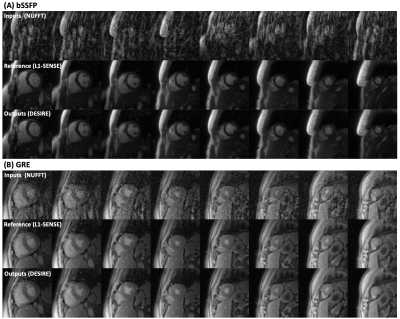
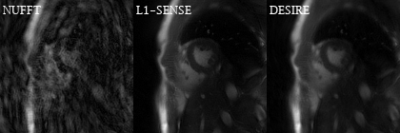
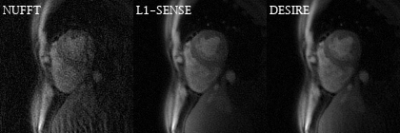
Excellent reconstruction performance using the proposed DESIRE technique was demonstrated (Table 1). Figure 2 shows examples from the testing data for the bSSFP (Figure 2-A) and GRE (Figure 2-B) imaging. The case shown in Figure 2-A had an image quality score of 4.5 for L1-SENSE and 3.5 for DESIRE. The case shown in Figure 2-B had an image quality score of 4 for both L1-SENSE and DESIRE.Video 1 shows an example case from a patient undergoing bSSFP imaging. The image quality scores of L1-SENSE and DEISRE were 4.5 and 4.5, respectively.
Video 2 shows comparisons of an example case from a patient undergoing GRE imaging. The image quality score of L1-SENSE and DEISRE were 5 and 5, respectively.
The reconstruction time was ~800 ms per image series with 40 frames on a NVIDIA Tesla P100 GPU, while the reconstruction time of L1-SENSE with 30 iterations on an Intel Xeon CPU (2.40 GHz) with GPU-accelerated NUFFT was ~20 minutes per slice. The pre-processing time was around 2 minutes for each image series.
Discussion and Conclusion
The proposed deep learning-based image reconstruction technique (DESIRE) enabled rapid and high-quality image reconstruction for both bSSFP and GRE high-resolution spiral real-time cine imaging at 1.5 T, showing great potential of advancing clinical workflow and improving diagnostic efficiency.Acknowledgements
This work was supported by NIH R01 HL131919 and Wallace H. Coulter Foundation Grant.References
1. Feng L, Srichai MB, Lim RP, et al. Highly accelerated real-time cardiac cine MRI using k–t SPARSE-SENSE. Magnetic Resonance in Medicine 2013;70:64–74.
2. Kühl HP, Spuentrup E, Wall A, et al. Assessment of Myocardial Function with Interactive Non–Breath-hold Real-time MR Imaging: Comparison with Echocardiography and Breath-hold Cine MR Imaging. Radiology 2004;231:198–207.
3. Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magnetic Resonance in Medicine 2004;51:93–102.
4. Lee VS, Resnick D, Bundy JM, Simonetti OP, Lee P, Weinreb JC. Cardiac Function: MR Evaluation in One Breath Hold with Real-time True Fast Imaging with Steady-State Precession. Radiology 2002;222:835–842.
5. Uecker M, Zhang S, Voit D, Karaus A, Merboldt K-D, Frahm J. Real-time MRI at a resolution of 20 ms. NMR Biomed. 2010;23:986–994.
6. Xue H, Kellman P, LaRocca G, Arai AE, Hansen MS. High spatial and temporal resolution retrospective cine cardiovascular magnetic resonance from shortened free breathing real-time acquisitions. J Cardiovasc Magn Reson 2013;15:102.
7. Kaji S, Yang PC, Kerr AB, et al. Rapid evaluation of left ventricular volume and mass without breath-holding using real-time interactive cardiac magnetic resonance imaging system. Journal of the American College of Cardiology 2001;38:527–533.
8. Zhou R, Yang Y, Mathew RC, et al. Free-breathing cine imaging with motion-corrected reconstruction at 3T using SPiral Acquisition with Respiratory correction and Cardiac Self-gating (SPARCS). Magnetic Resonance in Medicine 2019;82:706–720.
9. Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magnetic Resonance in Medicine 2010;64:767–776.
10. Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magnetic Resonance in Medicine 2014;72:707–717.
11. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magnetic Resonance in Medicine 2000;43:682–690.
12. Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Transactions on Signal Processing 2003;51:560–574.
13. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. arXiv:1505.04597 [cs] 2015.
14. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magnetic Resonance in Medicine 2000;43:682–690.
15. Cole E, Cheng J, Pauly J, Vasanawala S. Analysis of deep complex-valued convolutional neural networks for MRI reconstruction and phase-focused applications. Magnetic Resonance in Medicine 2021;00:1–17.
16. Zhou Wang, Bovik AC, Sheikh HR, Simoncelli EP. Image quality assessment: from error visibility to structural similarity. IEEE Transactions on Image Processing 2004;13:600–612.
Figures