0020
Free-running isotropic whole-heart T2 mapping with ECG-free Pilot Tone navigation
Simone Rumac1, Christopher W. Roy1, Jérôme Yerly1, Mariana B. L. Falcao1, Aurélien Bustin1,2,3, Mario Bacher1,4, Peter Speier4, Matthias Stuber1,5, and Ruud B. van Heeswijk1
1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2IHU LIRYC, Electrophysiology and Heart Modeling Institute, INSERM U1045, Centre de recherche Cardio-Thoracique de Bordeaux, Université de Bordeaux, Bordeaux, France, 3Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 4Siemens Healthcare GmbH, Erlangen, Germany, 5CIBM Center for BioMedical Imaging, Lausanne, Switzerland, Lausanne, Switzerland
1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2IHU LIRYC, Electrophysiology and Heart Modeling Institute, INSERM U1045, Centre de recherche Cardio-Thoracique de Bordeaux, Université de Bordeaux, Bordeaux, France, 3Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 4Siemens Healthcare GmbH, Erlangen, Germany, 5CIBM Center for BioMedical Imaging, Lausanne, Switzerland, Lausanne, Switzerland
Synopsis
The most commonly employed T2 mapping techniques are 2D and make use of ECG-triggering. This may be a limitation in patients with variable heart rate and complex three-dimensional conditions. To address these limitations, we here propose an isotropic free-running 3D T2 mapping technique that avoids ECG triggering by using Pilot Tone navigation. In three healthy volunteers, our technique produced accurate isotropic T2 maps when compared to 2D T2 prepared bSSFP (T2=41.1±4.8ms vs. 44.9±3.3ms, respectively, p=0.1), and cardiac motion was successfully resolved.
Introduction
Cardiac T2 mapping is routinely used in clinical MR protocols for the assessment of acute edema.1 Most commonly employed T2 mapping techniques are breath-held single-slice 2D acquisitions. This coverage can limit the sensitivity, especially in the case of myocardial pathologies that have heterogeneous 3D patterns throughout the myocardium. To more completely characterize these spatially complex disease patterns, several 3D T2 mapping techniques have therefore been proposed.2–6 Moreover, all current 2D and 3D T2 mapping techniques make use of electrocardiogram (ECG) triggering, which may lead to unpredictable scan times, depends on high-quality ECG signals, may fail in case of arrhythmia, and implies a time-inefficient sampling of k-space. Most of these limitations might be avoided by using a free-running acquisition with retrospective self-gating,7 but robust ECG-free self-gating is very challenging when a wide range of image contrasts is used, as is the case for mapping. To address these limitations, we here propose an isotropic free-running 3D T2 mapping technique that 1) utilizes Pilot Tone8 navigation to obtain contrast-independent respiratory and cardiac signals, 2) robustly corrects for respiratory motion through focused navigation (fNAV),9 3) fully resolves cardiac motion, 4) produces accurate maps through compressed sensing (CS) combined with patch-based denoising (HD-PROST).10Methods
The acquisition (Figure 1) consists of a prototype free-running7 3D radial GRE sequence with a phyllotaxis trajectory,11 field of view=(220mm)3, isotropic spatial resolution=(1.5mm)3, TR/TE=4.4/2.2ms, α=5°, four interleaved T2 preparation times T2-prep=0/25/40/55ms, 35200 readouts/T2-prep, and a total acquisition time of 11.3min. All data were acquired on a 1.5T clinical scanner (MAGNETOM Sola, Siemens Healthcare, Erlangen, Germany). Data-synchronized respiratory and cardiac signals were extracted from the Pilot Tone signal.12 The respiratory motion was corrected using focused navigation (fNAV),9 which estimates the amplitude of respiratory motion along all three spatial dimensions in order to scale the respiratory motion signals obtained from the Pilot Tone, and translationally corrects each k-space readout prior to the CS reconstruction, thus reducing the dimensionality of the dataset. Source images were reconstructed with total variation (TV) regularization along the cardiac dimension, and denoising was subsequently performed by applying HD-PROST to improve the T2 precision. Extended phase graph (EPG)13 simulations were used to generate a sequence-specific dictionary for a range of T2 values. A pixel-wise match of the source images with this dictionary then resulted in the T2 map. The proposed technique was validated in an agar-NiCl2-gel phantom designed to mimic the in-vivo range of myocardial relaxation times. Gold-standard values were obtained using a turbo-spin-echo (TSE) sequence with 32 echo times (13-422ms) and TR=10s. Linear regression and Bland-Altman analyses were performed to compare our technique and routine breath-held T2-prepared T2 mapping14 to the TSE relaxation times. Finally, both the proposed technique and the clinical routine scans were applied to the heart of three healthy volunteers (age=33±8y, 1F) with IRB approval and written informed consent. The myocardial T2 maps were manually segmented, and a Student’s t-test was used to compare the average values in the corresponding myocardial area to routine T2-prepared mapping.14 We measured the average myocardial T2 in all cardiac phases and computed its standard deviation as a measure of precision.Results
The phantom T2 maps demonstrated a high correlation with the gold standard over the relevant T2 range (y=0.98x-4.6, R2=0.98, Fig.2). A small (~6ms) underestimation was observed compared to the TSE T2 values, which was consistent in the range of interest. The Bland-Altman analysis confirmed the bias but did not reveal other trends. In all in-vivo cases, and despite the periodic interference produced by the T2-prep modules, Pilot Tone allowed a robust detection of both cardiac and respiratory signals. The fNAV correction successfully aligned the respiratory phases as evidenced by sharp lung-liver interfaces. The CS-based reconstruction produced well-aligned source images with resolved cardiac motion. The resulting motion-resolved T2 maps were accurate when compared to routine 2D maps (T2=41.1±4.8ms vs. 44.9±3.3ms, respectively, p=0.1). Moreover, we found that the average T2 value remained coherent across the cardiac cycle (Figure 4), with the average standard deviation across all phases being lower than 3ms for all considered subjects.Discussion
The proposed technique showed high correlation with the gold-standard TSE acquisition in the phantom, but a small T2 underestimation, which is mostly likely due to stimulated echoes in the TSE and is consistent with what was previously found in dictionary-matching-based techniques.15 The use of Pilot Tone allows for robust identification of physiological signals regardless of periodic changes in source image contrast. The fNAV correction proved to be a useful tool in order to reduce dimensionality and therefore reduced the computational burden while retaining all information. Finally, the T2 maps demonstrated high consistency across the different cardiac phases, albeit in a small sample size. Therefore, further optimization is required to improve both accuracy and precision in a larger cohort. In conclusion, we successfully demonstrated the feasibility and preliminary results of an isotropic free-running 3D T2 mapping technique.Acknowledgements
No acknowledgement found.References
- Messroghli, D. R. et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 19, 75 (2017).
- van Heeswijk, R. B. et al. Self-navigated isotropic three-dimensional cardiac T2 mapping. Magn. Reson. Med. 73, 1549–54 (2015).
- Ding, H. et al. Three-dimensional whole-heart T2 mapping at 3T. Magn. Reson. Med. 74, 803–16 (2015).
- Milotta, G. et al. 3D Whole-heart free-breathing qBOOST-T2 mapping. Magn. Reson. Med. 83, 1673–1687 (2020).
- Bustin, A. et al. Accelerated free‐breathing whole‐heart 3D T 2 mapping with high isotropic resolution. Magn. Reson. Med. 83, 988–1002 (2020).
- Qi, H. et al. Free-running simultaneous myocardial T1/T2 mapping and cine imaging with 3D whole-heart coverage and isotropic spatial resolution. Magn. Reson. Imaging 63, 159–169 (2019).
- Di Sopra, L., Piccini, D., Coppo, S., Stuber, M. & Yerly, J. An automated approach to fully self-gated free-running cardiac and respiratory motion-resolved 5D whole-heart MRI. Magn. Reson. Med. 82, 2118–2132 (2019).
- Speier, P., Fenchel, M. & Rehner, R. PT-Nav: A Novel Respiratory Navigation Method for Continuous Acquisition Based on Modulation of a Pilot Tone on the MR-Receiver. Magn. Reson. Mater. Phys. Biol. Med. 28, 1–135 (2015).
- Roy, C. W. et al. Motion compensated whole-heart coronary cardiovascular magnetic resonance angiography using focused navigation (fNAV). J. Cardiovasc. Magn. Reson. 23, 33 (2021).
- Bustin, A. et al. High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI. Magn. Reson. Med. 81, 3705–3719 (2019).
- Piccini, D., Littmann, A., Nielles-Vallespin, S. & Zenge, M. O. Spiral phyllotaxis: the natural way to construct a 3D radial trajectory in MRI. Magn. Reson. Med. 66, 1049–1056 (2011).
- Falcão, M. B. L. et al. Pilot tone navigation for respiratory and cardiac motion-resolved free-running 5D flow MRI. Magn. Reson. Med. (2021) doi:10.1002/mrm.29023.
- Weigel, M. Extended phase graphs: Dephasing, RF pulses, and echoes - Pure and simple. J. Magn. Reson. Imaging 41, 266–295 (2015).
- Giri, S. et al. T2 quantification for improved detection of myocardial edema. J. Cardiovasc. Magn. Reson. 11, 56 (2009).
- Hamilton, J. I. et al. Simultaneous Mapping of T1 and T2 Using Cardiac Magnetic Resonance Fingerprinting in a Cohort of Healthy Subjects at 1.5T. J. Magn. Reson. Imaging (2020) doi:10.1002/jmri.27155.
Figures
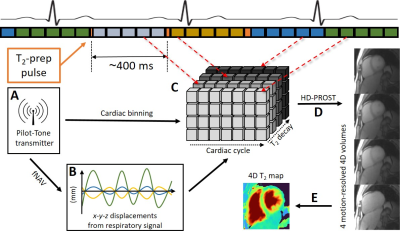
Overview of the proposed technique. A continuous 3D radial
GRE acquisition is periodically interleaved with T2-prep pulses of increasing duration. A) The Pilot Tone coil is used to obtain
respiratory and cardiac signals. B) The extracted signal is converted into a correction factor (fNAV) prior to reconstruction. C) A CS reconstruction is
performed with resolved cardiac and T2-prep dimensions. D) HD-PROST is applied to denoise the images, thus
obtaining four cardiac-motion-resolved 4D volumes with T2-weighted contrast. E) Dictionary matching is
used to create a 4D T2
map.
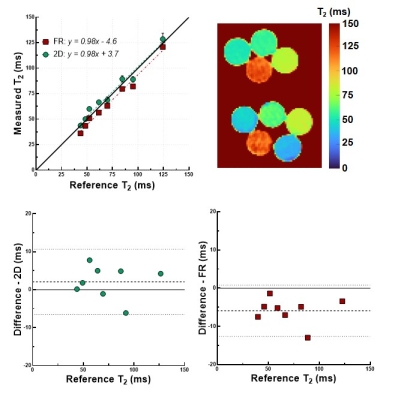
Validation
of the technique in the phantom. A) Linear regression plot of the T2 values obtained with the proposed technique.
The T2 relaxation
times show a slight but consistent underestimation when compared to the turbo-spin-echo
reference over the entire range of interest (35ms-130ms). B) Bland-Altman
analysis of routine 2D T2 mapping. C) Bland-Altman analysis of the proposed
technique, confirming the ~6ms bias. The dotted lines represent the 95% limits
of agreement.
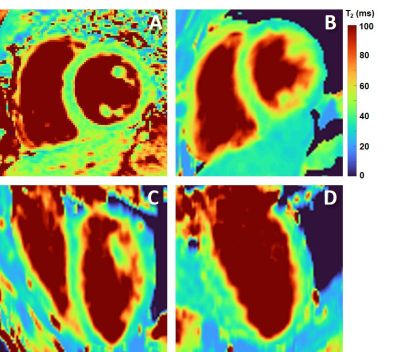
The
resulting maps compared to 2D T2-prepared bSSFP (M, 41 y.o). A) Short-axis breath-held 2D T2 map acquired with routine T2-prepared bSSFP. B) Short-axis slice obtained
with the proposed technique in mid-diastole. C-D) The corresponding orthogonal planes
(pseudo-4CH and pseudo-2CH).
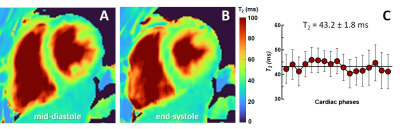
Average myocardial T2 across the cardiac cycle (M, 41 y.o). A) Mid-diastolic T2 map. B) Corresponding T2 map in the end-systolic phase. C) The average T2 measured in the segmented myocardial area.
The bars indicate the measured standard deviation.
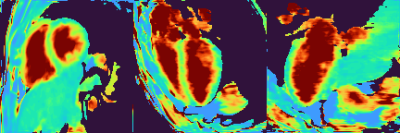
The resulting cardiac-resolved T2 map (M, 41 y.o). One short-axis, pseudo-4CH and pseudo-2CH slices across the full cardiac cycle.
DOI: https://doi.org/10.58530/2022/0020