0015
Heartbeat-resolved cardiac CINE imaging via motion corrected reconstructions1King's College London, London, United Kingdom
Synopsis
Conventional cardiac CINE imaging acquires segmented data over multiple heartbeats to satisfy sampling requirements. Recently, cardiac CINE reconstructions from a single heartbeat have been achieved using motion corrected reconstructions. This approach allows single heartbeat CINE and therefore can image the unique dynamics that occur in each heartbeat. Experiments in healthy subjects demonstrate that single heartbeat CINE is feasible and can detect heartbeat to heartbeat variation; the dynamics of individual heartbeats differ and cannot be detected with conventional CINE performed over multiple heartbeats. Single heartbeat CINE shows promise to characterize arrhythmias and other heart conditions where heartrate variability occurs.
INTRODUCTION:
Cardiac CINE imaging is usually acquired over multiple heartbeats such that enough data is acquired for each cardiac phase. This acquisition strategy assumes that the cardiac motion is periodic with only negligible differences between heartbeats. Several motion resolved, Compressed Sensing based reconstructions1,2,3 have been developed to reduce acquisition times, which nonetheless remain longer than one heartbeat and thus still assume cardiac motion periodicity. Recently, we proposed a Motion Corrected CINE (MC-CINE)4 approach, which enables cardiac CINE MRI from a single heartbeat. This method relies on non-rigid cardiac motion corrected reconstructions5,6,7 with low-rank patch-based regularization (PROST).8 The MC-CINE framework estimates the non-rigid cardiac motion from the acquired data itself and incorporates that information into the reconstruction of each cardiac phase, enabling single heartbeat CINE. We hypothesize that, by imaging each heartbeat separately, we more accurately depict motion dynamics that may vary between heartbeats. For example, arrhythmic events may be better characterized if only data from those heartbeats are used for reconstruction. The potential for single heartbeat imaging using MC-CINE was investigated in five healthy subjects and compared to conventional iterative SENSE (itSENSE)9 and XD-GRASP reconstructions.1METHODS:
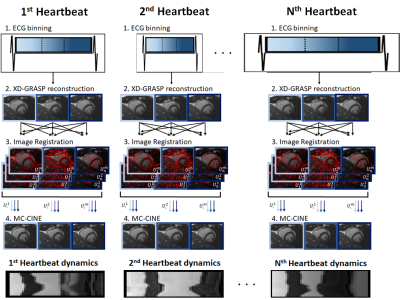
The MC-CINE framework involves four steps: 1) ECG-binning; 2) auxiliary XD-GRASP reconstruction; 3) non-rigid cardiac motion estimation via image registration; and 4) motion corrected reconstruction for each cardiac frame (Fig.1). Data from each heartbeat is equally divided into multiple cardiac phases and reconstructed with XD-GRASP. These preliminary images are then used for motion estimation via image registration.10,11 The final Motion Corrected CINE reconstruction is obtained by solving: $$ \hat{x} = \mathit{argmin}_{x} \left \| W_n\left ( \sum _n A_n F C M_n x - k \right ) \right \|_2 ^2 + \lambda \sum_b \left \| \tau _b \right \| _* s.t. \tau _b = Q_b\left ( \left ( M_n \right )^H x \right ) $$, where $$$ W_n $$$ are soft-weights for cardiac phase n, $$$ A_n $$$ is the corresponding sampling trajectory, $$$ F $$$ is the Fourier transform, $$$ C $$$ are coil sensitivities, $$$ M_n = [U_n^1, ... U_n^m]^T $$$ are the motion fields $$$ U $$$ from each cardiac phase m towards every cardiac phase n, $$$ x = [x^1, ... x^m]^T $$$ are the motion corrected CINE images for each cardiac phase, $$$ k $$$ is the acquired k-space data and $$$ Q_b $$$ assembles a 3D PROST tensor from non-rigidly aligned cardiac phases.EXPERIMENTS:
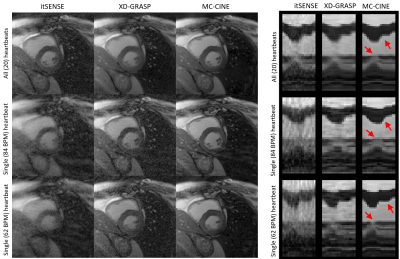
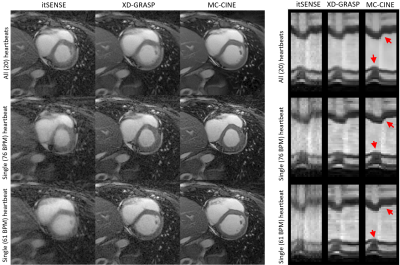
Five healthy subjects were scanned on a 1.5T scanner (Philips Ingenia). Imaging parameters included field of view (FOV) = 256x256 mm2; 8 mm slice thickness; resolution = 2x2 mm2; TE/TR = 1.16/2.3 ms; radial tiny golden angle; flip angle 60º; 8960 radial spokes acquired; nominal scan time ~20s; breath-hold acquisition. CINE images composed of 20 cardiac phases were reconstructed via iterative SENSE, XD-GRASP and MC-CINE. For each method, cardiac CINE images were reconstructed using: (1) all acquired data (20 heartbeats), (2) the single fastest heartbeat (acceleration factor ~ 24x), and (3) the single slowest heartbeat (acceleration factor ~ 20x).RESULTS:
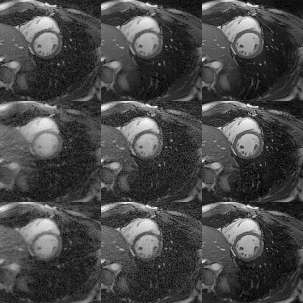
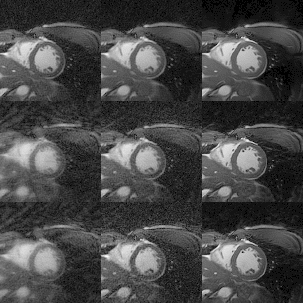
Cardiac CINE images and 1D+t profiles for two representative subjects are shown in Fig. 2 and Fig. 3, reconstructed using itSENSE, XD-GRASP and MC-CINE, using all acquired data (20 heartbeats), only data from the fastest heartbeat, and only data from the slowest heartbeat. In these Figures we can observe that the same cardiac phase can correspond to different cardiac motion states, depending on the heartbeat. Moreover, the 1D+t profiles present different dynamics for the slowest and the fastest heartbeat. While several artefacts arise in the reconstruction of heartbeat-resolved CINE using XD-GRASP and (especially) itSENSE, these artefacts are considerably reduced with MC-CINE. Animated CINEs of two additional subjects are shown in Fig. 4 and Fig.5. We can observe that while cardiac motion is correctly resolved with all these methods, itSENSE and XD-GRASP fail to produce Single heartbeat CINE devoid of artefacts and/or noise amplification, in contrast to the proposed MC-CINE. A comparison of the fastest and slowest heartbeat reconstructions indicates different motion dynamics between those.CONCLUSION:
A recently proposed Motion Corrected CINE (MC-CINE) reconstruction regularized by a non-rigidly aligned patch-based denoiser is employed to produce single heartbeat CINE. This approach does not share data between different heartbeats, therefore allowing us to image the non-periodic dynamics of cardiac motion. We expect such a method will be helpful to characterize arrhythmias and other conditions with heartrate variabilities. Future work will evaluate the MC-CINE framework in a patient cohort.Acknowledgements
This work was supported by EPSRC (EP/P001009,
EP/P032311/1, EP/P007619/1) and Wellcome EPSRC Centre for Medical Engineering
(NS/ A000049/1).
References
1. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD‐GRASP: golden‐angle radial MRI with reconstruction of extra motion‐state dimensions using compressed sensing. Magnetic resonance in medicine. 2016 Feb;75(2):775-88.
2. Lingala S, Hu Y, Dibella E, Jacob M (2011) Accelerated dynamic MRI exploiting sparsity and low-rank structure: K-t SLR. IEEE Trans Med Imaging 30(5):1042–1054. https://doi.org/10.1109/TMI.2010.2100850
3. Royuela-del Val J, Cordero-Grande L, Simmross-Wattenberg F, Martín-Fernández M, Alberola-López C (2017) Jacobian weighted temporal total variation for motion compensated compressed sensing reconstruction of dynamic MRI. Magn Reson Med 77(3):1208–1215. https://doi.org/10.1002/mrm.26198
4. Cruz G, Hammernik K, Kuestner T, Rueckert D, Botnar RM, Prieto C. One-heartbeat cardiac CINE imaging via jointly regularized non-rigid motion correct ed reconstruction. ISMRM 2021, abstract number 70.
5. Batchelor PG, Atkinson D, Irarrazaval P, Hill DL, Hajnal J, Larkman D. Matrix description of general motion correction applied to multishot images. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2005 Nov;54(5):1273-80.
6. Odille F, Vuissoz PA, Marie PY, Felblinger J. Generalized reconstruction by inversion of coupled systems (GRICS) applied to free‐breathing MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008 Jul;60(1):146-57.
7. Cruz G, Atkinson D, Henningsson M, Botnar RM, Prieto C. Highly efficient nonrigid motion‐corrected 3D whole‐heart coronary vessel wall imaging. Magnetic resonance in medicine. 2017 May;77(5):1894-908.
8. Bustin A, Ginami G, Cruz G, Correia T, Ismail TF, Rashid I, Neji R, Botnar RM, Prieto C. Five‐minute whole‐heart coronary MRA with sub‐millimeter isotropic resolution, 100% respiratory scan efficiency, and 3D‐PROST reconstruction. Magnetic resonance in medicine. 2019 Jan;81(1):102-15.
9. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k‐space trajectories. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2001 Oct;46(4):638-51.
10. Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE transactions on medical imaging. 1999 Aug;18(8):712-21. 11. Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, Fox NC, Ourselin S. Fast free-form deformation using graphics processing units. Computer methods and programs in biomedicine. 2010 Jun 1;98(3):278-84.
Figures