0012
Temporal correlation of functional connectivity with cerebral blood flow and T2 relaxation time in the monkey brain following ischemic stroke1Emory University, Atlanta, GA, United States, 2Department of Radiology, Emory University, Atlanta, GA, United States
Synopsis
Somatosensory deficits are seen in most stroke patients and can improve during recovery from stroke as reported previously. However, the underlining mechanism of the recovery remains poorly understood. The nonhuman primate (NHP) model of stroke has demonstrated similar functional connectivity evolution as seen in stroke patients and the secondary somatosensory cortex (S2) in NHPs is recognized as a direct extrapolation of somatosensory cortex in human. In the present study, the progressive function alteration of S2 and its relationship with the cerebral blood flow (CBF) and T2 changes in the monkey brain during acute stroke were examined and evaluated.
Introduction
Somatosensory impairment is seen in most stroke patients but has been underestimated in clinical practice because motor symptoms raise greater awareness in the clinic and preclinical studies of stroke [1, 2]. The secondary somatosensory cortex (S2) is known to involve the context of somatosensory dysfunction and sensory rehabilitation in stroke patients [1, 3]. However, the underlying mechanism remains poorly understood. The nonhuman primate (NHP) model of stroke has demonstrated similar functional connectivity (FC) evolution in S2 as seen in stroke patients [4]. In particular, the S2 in NHPs is recognized as a direct extrapolation of somatosensory cortex in human [5]. This study was aimed to investigate the functional alteration of S2 and its temporal relationship with regional cerebral blood flow (CBF) and T2 changes in the monkey brain during acute stroke.Methods
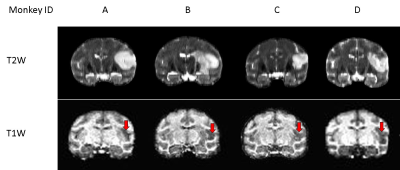
Permanent middle cerebral artery occlusion (MCAo) was induced in healthy adult rhesus monkeys (n = 4, 10-19 years old) using the minimally invasive interventional approach [6]. The resting state functional MRI (rsfMRI), CBF and T2 data were collected longitudinally before surgery (pre) and on Day 0, Day 2, and Day 4 post stroke on a 3T scanner. The rsfMRI data were collected with TR/TE=2190 ms/25ms, voxel size = 1.5 × 1.5 × 1.5 mm3 and 430 volumes. CBF was collected with the pseudo-arterial spin-labeling (pCASL) perfusion MRI with TR/TE = 3830ms /21ms, labeling-offset = 55 mm, post-labeling delay = 0.8 s, labeling duration= 2.0 s [7-9]. Multi-echo fast-spin echo sequence (TE=9.1, 64, 82 and 146 ms and TR=5780 ms) was used for data acquisition of T2 map calculation. T1 -weighted images were acquired for image co-registration and lesion identification (Fig.1). SPSS 26.0 was used for statistical analysis.The rsfMRI data preprocessing with the temporal filtering band-pass of 0.009 Hz ~0.0237 Hz were performed using a script from AFNI. Regions of interest (ROIs) of the contralateral (S2-Con) and ipsilateral (S2-Ipsi) S2 (Fig. 2) were defined based on the monkey brain MRI atlas[10] and corresponding T1 weighted images. The averaged time courses of the S2-Con were used as seed signals for seed-based correlation analyses. The relative connectivity (RelCon), a robust index of FC, was assessed in FC data analysis.
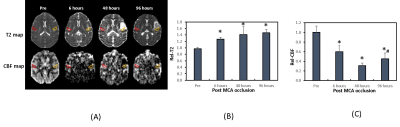
CBF and T2 data analyses were performed using home-built Matlab scripts [11] and ImageJ. Relative CBF (RelCBF) or T2 values (RelT2) were derived by using the contralateral S2 regions of the brain as reference (Fig. 3). Neurological score were assessed using the Spetzler approach including assessment of motor function, behavior, and ocular and cranial nerve function [12].
Analysis of variance (ANOVA) for repeated measures was performed to check the differences of the MRI measures across serial time points. The Pearson correlation was applied for correlation analysis.
Results
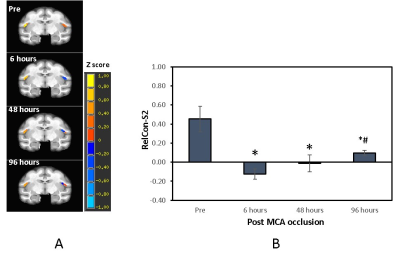
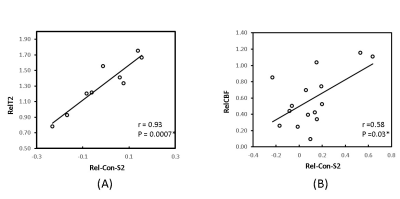
Cerebral infarction was observed in the MCA territory of the right hemisphere including the S2 region in each monkey (Fig.1). Substantial RelCon reduction was seen immediately following stroke insult on Day 0, but progressively increased on Day 2 and Day 4 post stroke (Fig. 2). The RelCon at Day 4 was significantly higher than that in Day 0 though it was still much lower than the pre-surgery result (Fig. 2). RelT2 progressively increased from Day 0 to Day 4 post stroke (Fig.3, A and B). Significant RelCBF reduction was seen from Day 0 to Day 4 post stroke (Fig.3, A and C). In addition, significant positive correlation was seen between RelCon-S2 and RelT2 from Day 0 to Day 4 post stroke (Fig. 4A) and between RelCon-S2 and RelCBF from pre to Day 4 post stroke (Fig. 4B). The Spetzler score dropped substantially on Day 0 but slightly recovery from Day 2 to Day 4 and was not correlated with the FC in S2 (p>0.4).Discussion
The preliminary rsfMRI results revealed progressive increase in FC of S2 from Day 0 to Day 4 post occlusion, indicating the spontaneous recovery process of S2 started before 48 hours post stroke[4]. The FC finding may partly explain why somatosensory deficits improved in patients mostly within the first 3 months after stroke insult [1]. In addition, our results showed the functional recovery in S2 temporally correlated with the evolution of the brain edema during acute stroke, indicating the functional recovery in S2 was spontaneously progressing despite the concurrent infarct and edema evolution. In addition, the preliminary results showed the S2 FC was temporally correlated with the regional CBF during the recovery while CBF at S2 was maintained at 30-50% of the normal ranges post stroke. As FC is closely coupled with CBF in the brain, the results in the monkey model may suggest the critical role of regional CBF during the S2 functional recovery in the first 96 hours post stroke [14].Conclusion
The preliminary results of a monkey model of stroke revealed the spontaneous function recovery process of S2 might start before 48 hours post MCA occlusion and kept improving significantly at 96 hours despite the on-going evolution of infarction and brain edema during acute stroke. Such FC finding may contribute to the interpretation of the improvement of somatosensory deficits in stroke patients.Acknowledgements
No acknowledgement found.References
1. Kessner, S.S., et al., Somatosensory Deficits After Ischemic Stroke. Stroke, 2019. 50(5): p. 1116-1123.
2. Kim, J.S. and S. Choi-Kwon, Discriminative sensory dysfunction after unilateral stroke. Stroke, 1996. 27(4): p. 677-82.
3. Lamp, G., et al., Activation of Bilateral Secondary Somatosensory Cortex With Right Hand Touch Stimulation: A Meta-Analysis of Functional Neuroimaging Studies. Front Neurol, 2018. 9: p. 1129.
4. Chun-Xia Li, F.T., Doty Kempf, Leonard Howell, Xiaodong Zhang, Temporal correlation of functional connectivity and Choline in the monkey brain following ischemic stroke. ISMRM (2021) 2021.
5. Bretas, R.V., et al., Secondary somatosensory cortex of primates: beyond body maps, toward conscious self-in-the-world maps. Exp Brain Res, 2020. 238(2): p. 259-272.
6. Zhang, X., et al., Temporal evolution of ischemic lesions in nonhuman primates: a diffusion and perfusion MRI study. PLoS One, 2015. 10(2): p. e0117290.
7. Li, C.X. and X.D. Zhang, Evaluation of prolonged administration of isoflurane on cerebral blood flow and default mode network in macaque monkeys anesthetized with different maintenance doses. Neuroscience Letters, 2018. 662: p. 402-408.
8. Li, C.X. and X.D. Zhang, Effects of Long-Duration Administration of 1% Isoflurane on Resting Cerebral Blood Flow and Default Mode Network in Macaque Monkeys. Brain Connectivity, 2017. 7(2): p. 98-105.
9. Li, C.X., et al., Effect of high dose isoflurane on cerebral blood flow in macaque monkeys. Magnetic Resonance Imaging, 2014. 32(7): p. 956-960.
10. K.S. Saleem, N.K.L., A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Academic Press, , London., 2007.
11. Li, C.X., et al., Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neurosci Lett, 2013. 541: p. 58-62.
12. Spetzler, R.F., et al., Chronic reversible cerebral ischemia: evaluation of a new baboon model. Neurosurgery, 1980. 7(3): p. 257-61.
13. Golestani, A.M., et al., Longitudinal evaluation of resting-state FMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair, 2013. 27(2): p. 153-63.
14. Jann, K., et al., Functional connectivity in BOLD and CBF data: similarity and reliability of resting brain networks. Neuroimage, 2015. 106: p. 111-22.
Figures