0009
Simultaneous lumen and vessel wall imaging with iT2Prep-BOOST for an efficient comprehensive assessment of aortic disease1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
Bright- and black-blood imaging are key contrasts for the assessment of lumen and vessel wall integrity in aortic MRI. These contrasts are usually acquired separately, resulting in long and inefficient examinations. Here we extend a previously introduced motion-compensated interleaved acquisition (iT2Prep-BOOST) to produce high-quality co-registered 3D bright- and black-blood images from a short predictable scan of ~8min. The proposed iT2Prep-BOOST approach resulted in bright-blood images with improved contrast compared to conventional bSFFP images, and black-blood images with increased volumetric coverage and resolution compared to conventional HASTE images, showing promise for a comprehensive assessment of aortic disease from a single scan.
Introduction
Cardiovascular MR has become the modality of choice for the assessment of aortic disease over the last decade, thanks to its lack of ionizing radiation exposure and high sensitivity and specificity, and superior soft tissue contrast compared to alternative imaging modalities such as computed tomography and transthoracic echocardiography1. Clinical guidelines suggest the acquisition of both bright-blood and black-blood MR imaging2 for the comprehensive assessment of aortic disease. High-quality imaging of the aortic lumen is relevant for patients with a wide spectrum of aortic pathologies3. Additionally, high-quality imaging of the aortic wall can enable the measurement of arterial wall thickness in different vascular territories, which has been shown to be useful in the prediction of adverse events in the presence of atherosclerosis4,5, anatomic description and biomechanical modelling in aneurysmal aortic disease6,7, and the prognostication post-coarctation of the aorta repair8.These two contrasts are usually acquired in separate scans. Bright-blood images are typically acquired under free-breathing using 3D contrast-enhanced (CE) or non-CE MR angiography sequences that rely on diaphragmatic navigators to minimize the effect of respiratory motion and enable multiplanar evaluation of the luminal thoracic aorta along with the underlying cardiovascular anatomy. Black blood imaging, that is generally acquired under breath-hold utilising 2D spin-echo sequences in different orientations, is utilised for evaluation of the aortic wall for hematoma or other causes of thickening, along with the quantification of plaque burden and detection of thrombus1,2. This approach results in prolonged scan times and potential mis-registration between images due to both patient motion and the different geometries and spatial resolution used for each scan.
In this study we address these issues by further accelerating and extending a previously introduced motion-compensated iT2Prep-BOOST sequence9. Using an interleaved scanning approach, the proposed sequence provides high-quality co-registered 3D bright and black-blood images for the assessment of aortic lumen and vessel wall from a short scan of ~8 min.
Methods
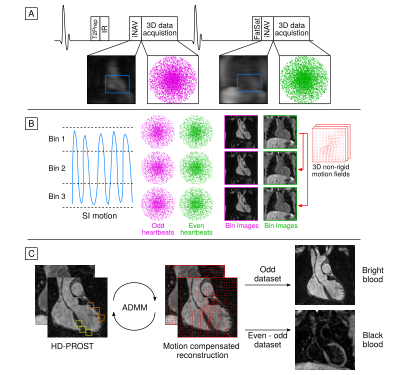
Data acquisition uses an ECG-triggered interleaved 3D balanced SSFP sequence (Fig 1a), where a T2Prep-IR module is applied before data acquisition in odd heartbeats and no preparation is applied in even heartbeats9. In both even and odd heartbeats 3D data is acquired with an undersampled variable-density Cartesian trajectory with spiral profile order10,11. 2D image-navigators (iNAVs) acquired at each heartbeat enable 100% respiratory efficiency without data rejection. Beat-to-beat 2D translational and bin-to-bin 3D non-rigid motion are estimated from the iNAVs and the 3D data itself (Fig 1b) and integrated into a non-rigid motion-compensated reconstruction with low-rank patch-based regularization (HD-PROST)12 (Fig 1c). The first bright-blood dataset can be used for lumen visualization, while subtraction of the two bright-blood datasets is used to create the black-blood dataset for vessel wall visualization.Fifteen patients with a variety of aortopathy (12 male, 29±7 years old) were scanned on a 1.5T system (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) using the proposed approach. 3D iT2Prep-BOOST images were acquired with the following parameters: coronal orientation, 4×undersampling, resolution=1.3mm3 isotropic, FOV=300×400×104-156mm3, T2Prep=40ms, TI=110ms, TE/TR=1.41/3.24ms, bandwidth=965 Hz/px, acquisition window=150ms. A 2D template was placed in the aortic arch to estimate the respiratory motion of the aorta from the 2D iNAVs. For assessment of the thoracic aorta, patients also underwent conventional bright-blood free-breathing diaphragmatic navigator gated 3D MR angiography (T2-prepared bSSFP sequence, sagittal orientation, resolution 1.4/1.5mm3 isotropic, FOV=240×400×134-168mm3, T2Prep=40ms) and 2D breath-held black-blood single shot fast spin echo (HASTE) (axial orientation, 1.56mm in-plane resolution, 8mm slice thickness, 23-36 slices, 3-5 breath-holds), with additional orientations acquired depending on each patients diagnosis. Scan time was recorded for all sequences.
Regions of interest were manually placed in the blood pool (sphere of 15 mm radius) and myocardium (sphere of 5 mm radius) in order to compute blood-to-myocardium contrast recovery coefficient (CRC) in the bright-blood images; while a visual comparison was performed between the conventional and proposed black-blood images.
Results
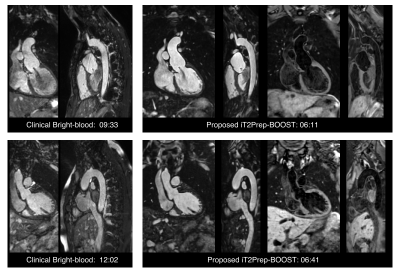
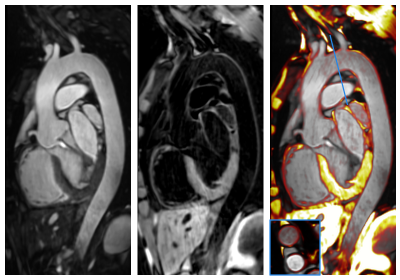
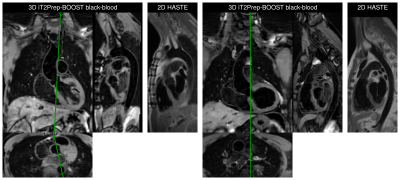
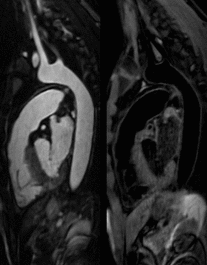
Average scan time for the conventional T2-prep bSSFP and HASTE sequences was 12.9±3.3min in total, while for the proposed 3D iT2Prep-BOOST approach was significantly shorter (7.9±1.8min, P<0.001), despite its higher spatial resolution. Visually improved quality of aortic luminal signal with attenuation of flow related artefacts can be observed with the proposed bright-blood technique (Fig 2), which also requires a shorter acquisition time and offers a complementary black-blood image for assessment of the vessel wall (Fig 3). Furthermore, a significantly increased CRC between blood and myocardium was observed for the proposed sequence compared with the conventional bright blood approach across patients (CRC T2-prep bSSPF: 9.2±2.1, CRC iT2Prep-BOOST: 15.2±4.2, P<0.001).When comparing black blood images, an improved coverage and resolution was observed for the black-blood 3D iT2Prep-BOOST compared to conventional 2D black-blood imaging (Fig 4). An animation through sagittal reformatting for a representative patient with the bright- and black-blood images obtained from the proposed iT2Prep-BOOST can be observed in Fig 5, showing excellent image quality for the depiction of thoracic aorta, holding promise for high-quality and reproducible measurements of the luminal caliber and assessment of the arterial wall thickness.
Conclusion
The proposed iT2Prep-BOOST approach provides excellent depiction of the aortic lumen and wall, with a predictable scan time of around 8 minutes, and holds promise for comprehensive assessment of aortic disease.Acknowledgements
This work was supported by the following grants: (1) EPSRC EP/P032311/1, EP/P007619/1, EP/P001009/1; (2) BHF programme grant RG/20/1/34802 and (3) Wellcome/EPSRC Centre for Medical Engineering (WT 203148/Z/16/Z).References
1. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease. J Am Coll Cardiol 2010;55:e27–129.
2. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020;22:17.
3. Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J 2014;35:2873–926.
4. Mani V, Muntner P, Gidding SS, Aguiar SH, Aidi H El, Weinshelbaum KB, et al. Cardiovascular magnetic resonance parameters of atherosclerotic plaque burden improve discrimination of prior major adverse cardiovascular events. J Cardiovasc Magn Reson 2009;11:1–12.
5. Bourque JM, Schietinger BJ, Kennedy JL, Pearce EA, Christopher JM, Taylor AM, et al. Usefulness of Cardiovascular Magnetic Resonance Imaging of the Superficial Femoral Artery for Screening Patients with Diabetes Mellitus for Atherosclerosis. Am J Cardiol 2012;110:50–6.
6. Shang EK, Nathan DP, Sprinkle SR, Fairman RM, Bavaria JE, Gorman RC, et al. Impact of wall thickness and saccular geometry on the computational wall stress of descending thoracic aortic aneurysms. Circulation 2013;128:157–62.
7. Shang EK, Lai E, Pouch AM, Hinmon R, Gorman RC, Gorman JH, et al. Validation of semiautomated and locally resolved aortic wall thickness measurements from computed tomography. J Vasc Surg 2015;61:1034–40.
8. Meyer AA, Joharchi MS, Kundt G, Schuff-Werner P, Steinhoff G, Kienast W. Predicting the risk of early atherosclerotic disease development in children after repair of aortic coarctation. Eur Heart J 2005;26:617–22.
9. Milotta G, Ginami G, Cruz G, Neji R, Prieto C, Botnar RM. Simultaneous 3D whole-heart bright-blood and black-blood imaging for cardiovascular anatomy and wall assessment with interleaved T2prep-IR. Magn Reson Med 2019;82:312–25.
10. Prieto C, Doneva M, Usman M, et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J Magn Reson Imaging 2015;41:738–46.
11. Bustin A, Ginami G, Cruz G, et al. Five-minute whole-heart coronary MRA with sub-millimeter isotropic resolution, 100% respiratory scan efficiency, and 3D-PROST reconstruction. Magn Reson Med 2019;81:102–115.
12. Bustin A, Lima da Cruz G, Jaubert O, Lopez K, Botnar RM, Prieto C. High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI. Magn Reson Med 2019;81:3705–3719.
Figures