0008
Wall shear stress and velocity pulsatility in the parent artery of an unruptured intracranial aneurysm - a 7T 4D flow MRI study.
Rick J. van Tuijl1, Ynte M. Ruigrok2, Kimberley M. Timmins1, Birgitta K Velthuis1, Pim van Ooij1, Jaco J.M. Zwanenburg1, and Irene C. van der Schaaf1
1Radiology, UMC Utrecht, Utrecht, Netherlands, 2Neurology, UMC Utrecht, Utrecht, Netherlands
1Radiology, UMC Utrecht, Utrecht, Netherlands, 2Neurology, UMC Utrecht, Utrecht, Netherlands
Synopsis
The influence of maximum wall shear stress (WSSMAX) and velocity pulsatility (vPI) are extensively investigated in aortic aneurysms, but its influence along the Circle of Willis (CoW) in patients with unruptured intracranial aneurysms (UIA) is unknown. Thirteen patients with an UIA were scanned at 7T-MRI using 4D phase-contrast MRI, comparing hemodynamic parameters between the parent artery of the UIA and the corresponding contralateral artery. The parent vessel of the UIA showed significantly higher WSSMAX, blood-flow and velocity, and lower vPI compared to the unaffected contralateral side. This study supports a hemodynamic role in the aetiology of aneurysm formation.
Introduction
An unruptured intracranial aneurysm (UIA) is a saccular dilatation of a brain vessel that is at risk of rupture. Aneurysm rupture results in an aneurysmal subarachnoid hemorrhage, a subtype of stroke with poor outcome1,2. The number of detected UIA is growing due to the increasing number and quality of brain scans. Timely treatment can prevent aneurysm rupture, but is mostly performed in UIAs with high rupture risk1.Prediction of aneurysm rupture is crucial in clinical decision making in patients with an UIA. Development and growth of an unruptured IA (UIA) is a multifactorial process with hemodynamic forces such as wall shear stress being an important contributor3. Arteries are under constant mechanical loading from the blood pressure and flow which causes endothelial shear stress and circumferential wall stress4. Novel imaging techniques as 7T-MRI 4D flow imaging enable accurate and reproducible measurement and quantification of blood flow and the resulting wall shear stress (WSS), blood-flow, mean velocity and velocity pulsatility index (vPI) in the intracranial vessels5. Since abnormal levels of WSS are implicated in the pathogenesis of aneurysmal disease6, relation between wall shear stress and presence of an UIA in a blood vessel may provide insight in the aetiology of UIA7. This study used accelerated 4D-flow imaging to compare the WSS, blood-flow, mean velocity and vPI in the intracranial parent artery containing an UIA with the corresponding contralateral artery without UIA.
Methods
This study included subjects from the 3T-MRI ERASE (Early Recognition of persons at high risk of Aneurysmal Subarachnoid hemorrhagE) study, a screening study for UIA in family members of patients with an UIA. We included patients with a detected UIA that underwent additional 7-Tesla with a four-dimensional phase-contrast-MRI (4D PC-MRI) acquisition. UIA locations were only included if there was a contralateral artery without an UIA and if the UIA was included in the 4D PC-MRI acquisition. Participants underwent 7-Tesla MRI (Philips Healthcare, Best, The Netherlands) using a volume transmit and 32-Channel receive coil (Nova Medical, Houston, United States). For this study, we used PROspective Undersampling in multiple Dimensions (PROUD) acceleration, which enables a pseudospiral ky/kz-plane acquisition scheme designed for incoherent undersampling with a variable sampling density8. The 4D PC-MRI acquisition covered the complete CoW with scan specifics as given in Table 1.The reconstructed 4D PC-MRI datasets were analysed using CAAS MR Solutions v5.1.1 software (Pie Medical Imaging, Maastricht, The Netherlands). CAAS automatically generates the centreline and perpendicular slices along the complete CoW from the ICA towards the smaller cerebral arteries. These perpendicular slices were visually checked and automatically propagated to create volumetric flow rate traces and separate velocity and area traces over the cardiac cycle.
The blood-flow velocity pulsatility index (vPI=(Velocitymax-Velocitymin)/Velocitymean) was calculated from each velocity trace. The maximum WSS (WSSMAX) was calculated using an in-house-developed software in Matlab R2016a by multiplying the wall shear rate by the dynamic viscosity of blood (3.2x10-3 Pa·s)9 (Figure 1). The outcome parameters WSSMAX, blood-flow, vPI and mean velocity over the cardiac cycle were calculated for all patients with UIAs (Figure 2) for two ROIs: 1. parent artery of the UIA, 2. the corresponding contralateral artery. An affected (ipsilateral) side versus unaffected (contralateral) side comparison was performed for each patient using paired t-test. The statistical significance threshold was set at p<0.05.
Results
We included 14 patients (nine women, mean age 52 ± 12) with UIA (range 1.5 to 5 mm). One patient with a posterior inferior cerebral artery (PICA)) outside the field-of-view was excluded. Thirteen patients were included with the following UIA locations: six middle cerebral artery; three internal carotid artery (ICA); two posterior communicating artery and two anterior cerebral artery). WSSMAX, blood-flow and mean velocity were statistically significantly higher, and the vPI was statistically significantly lower in the parent artery containing an UIA compared to the contralateral artery (Table 2). These results were consistent for all 13 included patients with different UIA locations.Discussion
This study using 4D PC-MRI on ultra-high field (7T) showed different hemodynamics in the parent artery containing an UIA compared to the contralateral side without an UIA. The WSSMAX, blood-flow, mean velocity and vPI are thus potentially of risk for aneurysm development, growth and/or rupture. The limitation of this study is the limited number of UIAs. All UIA were in the anterior circulation, and therefore no conclusions can be drawn regarding the posterior circulation. However, we showed significant differences in these relatively small diameters of the UIAs, and all measurements were different within the same subject. Future studies need to include a larger population, preferably with long term follow-up, with also larger UIA in both anterior- as well as posterior circulation, to study the relation between WSS, blood-flow, velocity and vPI in the parent vessels and the presence of an aneurysm.Conclusion
This study shows that hemodynamic changes occur in parent arteries with UIA compared to the contralateral artery without UIA. All outcome measurements (WSSMAX, blood-flow and vPI) show statistically significant differences. These three hemodynamic markers in the parent arteries with UIA should be investigated further in larger population with UIA.Acknowledgements
We thank the study participants for their participation and the support of The Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2015‐008 ERASE), Dutch Federation of University Medical Centers, The Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences.References
1. Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat Rev Neurol. 2016;12(12):699-713.2. Algra AM, Lindgren A, Vergouwen MDI, et al. Procedural Clinical Complications, Case-Fatality Risks, and Risk Factors in Endovascular and Neurosurgical Treatment of Unruptured Intracranial Aneurysms: A Systematic Review and Meta-analysis. JAMA Neurol. 2019;76(3):282-293.
3. Szajer J, Ho-Shon K. A comparison of 4D flow MRI-derived wall shear stress with computational fluid dynamics methods for intracranial aneurysms and carotid bifurcations — A review. Magn Reson Imaging. 2018;48:62-69.
4. Lu D, Kassab GS. Role of shear stress and stretch in vascular mechanobiology. J R Soc Interface. 2011;8(63):1379-1385.
5. Markl M, Frydrychowicz A, Kozerke S, et al. 4D flow MRI. J Magn Reson Imaging. 2012;36(5):1015-1036.
6. Dolan JM, Kolega J, Meng H. High Wall Shear Stress and Spatial Gradients in Vascular Pathology: A Review. Ann Biomed Eng. 2013;(1):1411-1427.
7. Paszkowiak JJ, Dardik A. Arterial wall shear stress: Observations from the bench to the bedside. Vasc Endovascular Surg. 2003;37(1):47-57.
8. Gottwald LM, Töger J, Markenroth Bloch K, et al. High spatiotemporal resolution 4D flow MRI of intracranial aneurysms at 7T in 10 minutes. Am J Neuroradiol. 2020;41(7):1201-1208.
9. Van Ooij P, Potters W V., Guédon A, et al. Wall shear stress estimated with phase contrast MRI in an in vitro and in vivo intracranial aneurysm. J Magn Reson Imaging. 2013;38(4):876-884.
Figures
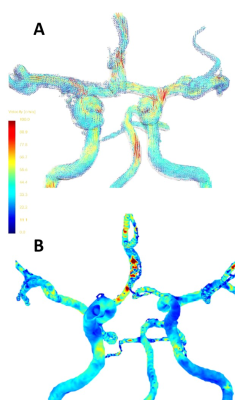
Figure 1: Representation of the steps from 4D flow images to the analysis, whereby the red arrow points to the aneurysm location in this subject. A) 4D PC-MRI images are reconstructed and visualized over the cardiac phase with velocity scaled between 0 and 100 cm/s in this subject. B) The wall shear stress distribution along the Circle of Willis used for analysis regarding affected (ipsilateral) versus unaffected (contralateral) side.
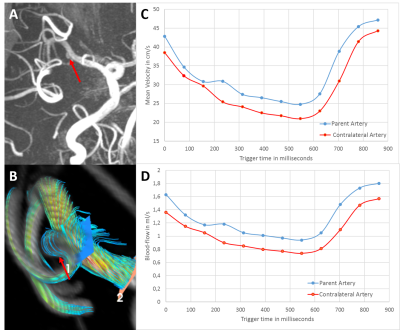
Figure 2: Representation of the 4D flow image analysis regarding affected (ipsilateral) versus unaffected (contralateral) side using CAAS software, whereby the red arrow points to the aneurysm location in this subject. A) MRA TOF image to show the aneurysm in this subject. B) Blood-flow analysis using the CAAS software for that same subject focused on the parent artery. C) Mean velocity curves (in cm/s) for this subject in the parent (blue) and contralateral artery (red). D) Blood-flow (in ml/s) for this subject in the parent (blue) and contralateral artery (orange).
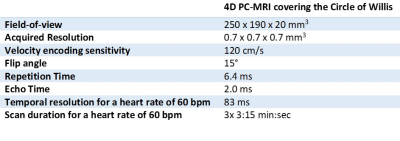
Table 1: Scan parameters.
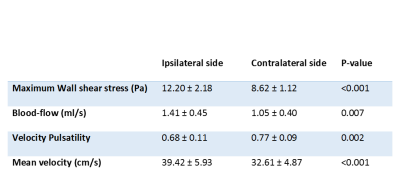
Table 2: Outcome parameters for all included subjects calculated at the ipsilateral side (parent artery), where the aneurysm is located, and the unaffected contralateral side.
DOI: https://doi.org/10.58530/2022/0008