0007
Defining Normative Cerebral Hemodynamics in Cognitively Healthy Older Adults with 4D Flow MRI1Dept of Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Dept of Radiology, University of Wisconsin - Madison, Madison, WI, United States, 3Dept of Medicine, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
It has been shown that vascular disease is strongly associated with Alzheimer’s disease (AD). It is thus important to establish normative cerebrovascular hemodynamics in aging populations. In this study, we comprehensively assess macrovascular hemodynamics using 4D flow MRI to obtain flow rates and pulsatility indices in 110 cognitively healthy, older adults and correlate these measures with age, sex, atherosclerotic cardiovascular disease (ASCVD) risk scores, and APOE genotypes. We found a (1) negative correlation between flow vs. age and flow vs. ASCVD, (2) a positive correlation between pulsatility vs. age and pulsatility vs. ASCVD, and (3) no correlations with APOE genotypes.
Introduction
Alzheimer’s disease (AD) is currently the sixth leading cause of death whose prevalence continues to grow due to demographic shifts and lack of treatments1. Recent evidence indicates that vascular pathology is a major risk factor for AD-related dementia with several studies indicating that it not only contributes to cognitive decline but also to neuronal loss in AD-related Aβ and tau pathologies2-5. Thus, there is considerable interest in defining cerebrovascular biomarkers for prodromal and advanced AD. 4D flow MRI enables a comprehensive assessment of intracranial hemodynamics in a single, whole-brain acquisition. While several 4D flow studies have investigated vascular dysfunction in AD6-9, normal 4D flow hemodynamic data in healthy older subjects is lacking. The primary aim of this study is to evaluate normative hemodynamic measures (specifically flow and pulsatility) in a large cohort of cognitively normal subjects using 4D flow MRI and to establish correlations with age, sex, atherosclerotic cardiovascular disease (ASCVD) risk score10, and APOE genotype.Methods
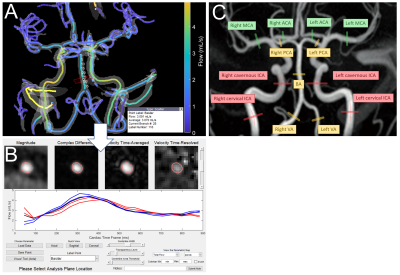
In this preliminary study, a sub-cohort of 110 subjects (73F/37M; mean age=67y; age range=[46-81y]) were selected from a larger cohort of older, cognitively normal subjects from the Wisconsin Alzheimer’s Disease Research Center. Inclusion criteria were defined as a normal cognitive status via comprehensive clinical diagnosis11 and a Pittsburgh Compound B index < 1.1912,13. Demographics, ASCVD risk scores, and APOE genotypes were obtained.4D flow MRI data were acquired at 3T (Signa Premier, GE Healthcare, WI) using a radially-undersampled PCVIPR14,15 acquisition with the following parameters: TR/TE=7.7/2.5ms; flip=8°; projections=11,000; isotropic resolution=0.69mm; image volume=22x22x10cm3; VENC=80cm/s; scan time=5.6 min; encode scheme=4-point. The data was reconstructed into 20 cardiac frames using retrospective peripheral pulse oximeter gating and temporal radial view sharing16. An interactive, semi-automated 4D flow processing tool (Figure 1A-B, available on Github) was developed in Matlab2020b (Mathworks, Natick, MA), which included automated vessel segmentation17, centerline detection, and reporting functions for a robust and user-independent analysis. Mean volumetric flow rates and pulsatility indices (PIs) were obtained in 15 major vessel segments: cervical internal carotid arteries (ICA), cavernous ICAs, vertebral arteries (VA), basilar artery (BA), middle cerebral arteries (MCA), posterior cerebral arteries (PCA), straight sinus (StrS), superior sagittal sinus (SSS), and transverse sinuses (TS). Total cerebral blood flow was computed as the sum of the cervical ICAs and BA. For bilateral vessels, left and right segments were averaged. Two observers separately analyzed 55 cases each, using standardized vessel measurement locations (Figure 1C).
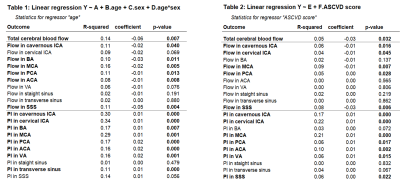
After 4D flow hemodynamic data had been collected, two simple linear regression models were used to (1) assess correlations between each outcome variable (flow and PI in each vessel) and age as well as (2) each outcome variable and ASCVD score. Multiple linear regression was then used to assess correlations between each outcome variable with age, sex, and age-sex interactions. Scatter plots were obtained for each regression model.
Results
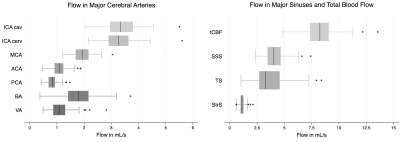
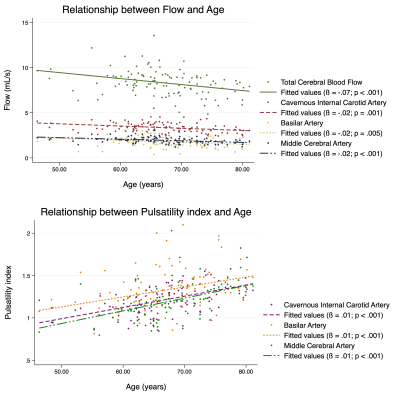
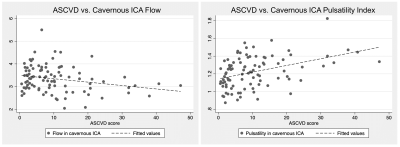
All 110 subjects were successfully processed, with analysis taking approximately 9 minutes for each case. Box plots for blood flow for all measured vessel segments (and total cerebral blood flow) are shown in Figure 2. Simple linear regression revealed that age is a predictor of decreased flow and increased PI in most vessel segments (Figure 3). For instance, age was positively correlated with flow (p = 0.001) and negatively correlated with PI (p < 0.001) in the cavernous ICA. In the multiple regression analysis, age showed the same relationship with flow and PI, but sex and the interaction between age and sex did not correlate significantly with flow or PI. ASCVD score was also found to be a predictor of decreased blood flow and increased PI for most vessel segments (Figure 3-4). Finally, flow and PI were not significantly correlated with APOE genotype.Discussion
It was observed that individual vessel flow rates and total cerebral blood flow decline with age (consistent with formerly published studies18-20), and that pulsatility increases with age. However, it should be noted that there may be low statistical power due to the limited sample size used in this study. Furthermore, blood flow values align well with those reported in other MRI21 and ultrasound22 studies. While APOE genotypes have been found to differentially alter cerebral blood flow using other MRI methods23, this was not observed in our study. We plan to continue analyzing normal subjects, providing a robust hemodynamic baseline. This is not only useful for future studies evaluating vascular dysfunction in mild cognitive impairment and AD but any cerebrovascular-related study interested in normal flow and pulsatility values.Conclusion
This preliminary investigation represents a first step towards defining normal cerebral blood flow and pulsatility values utilizing 4D flow MRI, which provides a sensitive, comprehensive and non-invasive tool for the assessment of cerebral luminal blood flow and pulsatility. Normal flow and pulsatility value, as have been reported in this study, show correlations with age and vascular risk scores and are an important first step in defining normative cerebral hemodynamics. Future studies will evaluate correlations with other vascular measures, such as white matter hyperintensities, as well as further improve 4D flow post-processing times.Acknowledgements
We gratefully acknowledge research support from GE Healthcare and funding support from the National Institutes of Health (F31-AG071183, KL2-TR002374, R01-AG027161).References
1. Alzheimer's A. 2016 Alzheimer's disease facts and figures. Alzheimers Dement 2016;12(4):459-509.
2. Stampfer MJ. Cardiovascular disease and Alzheimer's disease: common links. J Intern Med 2006;260(3):211-223.
3. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature Reviews Neuroscience 2011;12(12):723-738.
4. Corriveau RA, Bosetti F, Emr M, et al. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol Neurobiol 2016;36(2):281-288.
5. de la Torre J. The Vascular Hypothesis of Alzheimer's Disease: A Key to Preclinical Prediction of Dementia Using Neuroimaging. J Alzheimers Dis 2018;63(1):35-52.
6. Rivera-Rivera LA, Schubert T, Turski P, et al. Changes in intracranial venous blood flow and pulsatility in Alzheimer's disease: A 4D flow MRI study. J Cereb Blood Flow Metab 2017;37(6):2149-2158.
7. Rivera-Rivera LA, Turski P, Johnson KM, et al. 4D flow MRI for intracranial hemodynamics assessment in Alzheimer's disease. J Cereb Blood Flow Metab 2016;36(10):1718-1730.
8. Clark LR, Berman SE, Rivera-Rivera LA, et al. Macrovascular and microvascular cerebral blood flow in adults at risk for Alzheimer's disease. Alzheimers Dement (Amst) 2017;7:48-55.
9. Xu G, Rowley HA, Wu G, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer's disease. NMR Biomed 2010;23(3):286-293.
10. Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S49-73.
11. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7(3):263-269.
12. Lewczuk P, Riederer P, O'Bryant SE, et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J Biol Psychiatry 2018;19(4):244-328.
13. Tudorascu DL, Minhas DS, Lao PJ, et al. The use of Centiloids for applying [(11)C]PiB classification cutoffs across region-of-interest delineation methods. Alzheimers Dement (Amst) 2018;10:332-339.
14. Gu T, Korosec FR, Block WF, et al. PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR Am J Neuroradiol 2005;26(4):743-749.
15. Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med 2008;60(6):1329-1336.
16. Liu J, Redmond MJ, Brodsky EK, et al. Generation and visualization of four-dimensional MR angiography data using an undersampled 3-D projection trajectory. IEEE Trans Med Imaging 2006;25(2):148-157.
17. Schrauben E, Wahlin A, Ambarki K, et al. Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries. J Magn Reson Imaging 2015;42(5):1458-1464.
18. Buijs PC, Krabbe-Hartkamp MJ, Bakker CJ, et al. Effect of age on cerebral blood flow: measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology 1998;209(3):667-674.
19. Amin-Hanjani S, Du X, Pandey DK, Thulborn KR, Charbel FT. Effect of age and vascular anatomy on blood flow in major cerebral vessels. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2015;35(2):312-318.
20. Wu C, Honarmand AR, Schnell S, et al. Age-Related Changes of Normal Cerebral and Cardiac Blood Flow in Children and Adults Aged 7 Months to 61 Years. J Am Heart Assoc 2016;5(1).
21. Enzmann DR, Pelc NJ. Normal flow patterns of intracranial and spinal cerebrospinal fluid defined with phase-contrast cine MR imaging. Radiology 1991;178(2):467-474.
22. Scheel P, Ruge C, Petruch UR, Schöning M. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke 2000;31(1):147-150.
23. Wierenga CE, Clark LR, Dev SI, et al. Interaction of age and APOE genotype on cerebral blood flow at rest. Journal of Alzheimer's disease : JAD 2013;34(4):921-935.
Figures