0989
Motion Corrected Silent ZTE Neuroimaging
Emil Ljungberg1,2, Tobias C. Wood1, Ana Beatriz Solana3, Steven C.R. Williams1, Gareth J. Barker1, and Florian Wiesinger1,3
1Department of Neuroimaging, Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, United Kingdom, 2Department of Medical Radiation Physics, Lund University, Lund, Sweden, 3GE Healthcare, Munich, Germany
1Department of Neuroimaging, Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, United Kingdom, 2Department of Medical Radiation Physics, Lund University, Lund, Sweden, 3GE Healthcare, Munich, Germany
Synopsis
We present a new method called MERLIN for motion corrected silent neuroimaging using zero echo time (ZTE) MRI. Self-navigation is achieved with an interleaved 3D spiral phyllotaxis trajectory to produce image navigators. Retrospective motion correction is then applied to the collected raw data. The acoustic noise of MELIN was less than 4 dBA above ambient levels. A range of different head motion paradigms were evaluated (rotation and nodding), showing greatly improved image quality after motion correction in all cases.
Introduction
Acoustic noise and patient motion are two problems that needs to be addressed to further improve the value of MRI and to increase access to the most vulnerable patient populations.1,2 Motion artefacts can incur large costs to hospitals,2 and introduce confounds in research studies where it can bias morphological measurements.3 While the acoustic noise can be handled in most situations using hearing protection, it can be a problem for subjects with hypersensitivity to noise, and when scanning needs to be performed under natural sleep.Many solutions have been proposed to solve the problems of motion and acoustic noise separately. In this work we aim to solve these two problems together with a new method called MERLIN4 (Motion Estimation & Retrospective correction Leveraging Interleaved Navigators). MERLIN utilises Zero Echo Time (ZTE) imaging for silent data acquisition, together with an interleaved 3D radial trajectory for self-navigation and retrospective motion correction. We demonstrate the utility of MERLIN for T1-weighted neuroimaging with different types of head motion.
Methods
Self-navigated silent ZTEIn ZTE, data acquisition is performed with RF excitation in the presence of a gradient, followed by a rapid readout of a radial-out spoke in k-space. ZTE is thus well-suited for self-navigation as each k-space line, called spoke, originates from the centre of k-space. Silent self-navigation is achieved by splitting up a fully sampled spoke distribution into multiple spiral distributions, called interleaves (Figure 1A). The distance between subsequent spokes in an interleaf must be small, i.e., a smooth path, to maintain silent operation. Each interleaf is then reconstructed into a navigator image. We adopted the 3D spiral phyllotaxis k-space trajectory for this purpose due to its simple & elegant mathematical formulation.5 The spherical coordinates (ϕ azimuth, θ polar angle) of the endpoint of spoke i in interleaf j is given by:
$$\phi_{i,j}=(i⋅k+j)⋅\phi_G, \qquad i=0…N_s-1\\
z_{i,j}=1-(i⋅N_i+j)⋅Δz, \qquad Δz=\frac{2}{N_s⋅N_i-1},\\
\theta_{i,j}=acos(z_{i,j} ), \qquad j=0…N_i-1,$$
Where Ns is the number of spokes per interleaf, Ni the number of interleaves, and k is a Fibonacci number, which determines the sparsity of the spiral (Figure 1B & C), and thus also the acoustic noise and image quality (Figure 1D). In a steady-state ZTE phantom experiment we determined that k=55 and Ns~1024 delivered a reasonable compromise between navigator image quality and acoustic noise.
In vivo experiments
Three healthy volunteers were scanned under local ethics approval on a 3T GE MR750 (GE Healthcare, Waukesha, WI) with a T1-weighted ZTE sequence, using a 32-channel head receive coil (Nova Medical, Wilmington, MA). The acquisition parameters were: FOV=192x192x192 mm3, resolution=1x1x1 mm3, TI=450 ms, FA=3°, readout BW=±31.25 kHz, TR=1.8 ms, 384 acquired spokes per TI, and undersampling relative to Nyquist of 1.25. Each navigator was acquired with Ns=1152 spokes, with a duration of 3.5 ms (longer than in Figure 1D due to added inversion time).
The first samples along each spoke are missed in ZTE, called the deadtime gap. This was filled implicitly in each navigator using a conjugate gradient (CG) SENSE reconstruction with sensitivity maps obtained from an up-front WASPI acquisition.6,7 Navigator images were reconstructed using a sliding window with a length of 384 spokes, to increase the temporal resolution. Navigator registration was performed using a rigid body transform to the first navigator, and motion correction was then performed by applying a phase ramp to k-space data and rotation of the trajectory coordinates. The final image was reconstructed using an iterative SENSE approach with TGV regularisation.8 The complete MERLIN framework is shown in Figure 2.
A total of six scans were acquired for each subject, two static reference scans followed by four motion paradigms: big & small rotation, nodding, and continuous side-to-side motion. Image quality was compared relative to the first static reference scan using the Average Edge Strength (AES)9 and the mean Structural Similarity Index Measure (mSSIM).10 An open source implementation of the MERLIN framework is available on Github (github.com/emilljungberg/pyMERLIN) together with examples using simulated data (github.com/emilljungberg/merlin_mrm).
Results
The interleaved trajectory measured 4 dBA above ambient noise levels (Figure 1). Axial slices from subject 3 shown in Figure 3 demonstrate greatly improved image quality following motion correction with MERLIN for all motion paradigms, with up to 3 mm translation and 10° rotational motion. Figure 4 shows an example from subject 2 with larger motion, up to 20° rotation, where image quality was recovered after motion correction. Quantitative AES and mSSIM results (Figure 5) show that image quality was greatly improved for all subject after motion correction, further supporting the qualitative assessments.Discussion and Conclusion
We have presented MERLIN, a new method for retrospective motion correction of self-navigated ZTE for silent and motion robust neuroimaging. While the 3D radial sampling pattern makes ZTE less sensitive to motion, motion correction is nevertheless required to obtain good image quality in the presence of motion, as shown here. In this work we demonstrate MERLIN using T1w ZTE, but the framework can be applied to any image contrast including T2, T2*, QSM, angiography or synthetic CT for PET/MR and MR radiotherapy planning. Our hope is that MERLIN will increase the value of MRI in both clinical and research practice by creating a more patient-friendly environment and deliver good image quality in the presence motion for all patients.Acknowledgements
This work was supported by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z] and GE Healthcare. It represents independent research part funded by the NIHR-Wellcome Trust King’s Clinical Research Facility and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. F. Wiesinger and A.B. Solana are employees of General Electric Healthcare.References
- Sartoretti, E. et al. Impact of Acoustic Noise Reduction on Patient Experience in Routine Clinical Magnetic Resonance Imaging. Academic Radiology 0, 1–8 (2020).
- Slipsager, J. M. et al. Quantifying the Financial Savings of Motion Correction in Brain MRI: A Model-Based Estimate of the Costs Arising From Patient Head Motion and Potential Savings From Implementation of Motion Correction. Journal of Magnetic Resonance Imaging 1–8 (2020) doi:10.1002/jmri.27112.
- Reuter, M. et al. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. NeuroImage 107, 107–115 (2015).
- Ljungberg, E. et al. Motion Corrected Silent ZTE Neuroimaging. Magnetic Resonance in Medicine (2022) doi:10.1002/mrm.29201. (In production)
- Piccini, D., Littmann, A., Nielles-Vallespin, S. & Zenge, M. O. Spiral phyllotaxis: The natural way to construct a 3D radial trajectory in MRI. Magnetic Resonance in Medicine 66, 1049–1056 (2011).
- Wood, T. & Brackenier, Y. Implicit ZTE Dead-time Gap Filling with Iterative Reconstruction in Book of Abstracts ESMRMB 2021 Online 38th Annual Scientific Meeting 7–9 October 2021. in ESMRMB 2021, 38th Annual Scientific Meeting (In press) (2021). doi:10.1007/s10334-021-00947-8.
- Wu, Y. et al. Water- and fat-suppressed proton projection MRI (WASPI) of rat femur bone. Magnetic Resonance in Medicine 57, 554–567 (2007).
- Knoll, F., Bredies, K., Pock, T. & Stollberger, R. Second order total generalized variation (TGV) for MRI. Magnetic Resonance in Medicine 65, 480–491 (2011).
- Aksoy, M. et al. Hybrid prospective and retrospective head motion correction to mitigate cross-calibration errors. Magnetic Resonance in Medicine 67, 1237–1251 (2012).
- Wang, Z., Bovik, A. C., Sheikh, H. R. & Simoncelli, E. P. Image quality assessment: From error visibility to structural similarity. IEEE Transactions on Image Processing 13, 600–612 (2004).
Figures
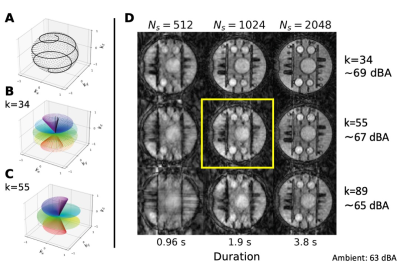
Figure 1: (A) Single spiral interleave (line) on top of a
full spoke distribution (points). (B) and (C) Examples of spiral interleaves
with Ns=384 spokes and different factors of k, controlling sparsity. (D)
Phantom image quality and noise levels for different interleave settings.
Ns=1024 and k=55 (yellow square) was determined as a good compromise between
image quality and acoustic noise. (Adapted with permission from Ljungberg et
al. 2022 [4] under a CC-BY license)
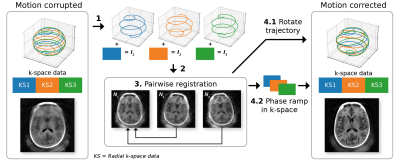
Figure 2: Framework for MERLIN
motion correction. (1) The acquired data is
separated into interelaves which are (2) reconstructed into navigator images.
(3) These are co-registered, followed by motion correction with a (4.1)
trajectory coordinate rotation for image rotations, and (4.2) k-space
phase ramp for translations. (Reproduced with permission from Ljungberg et al.
2022 [4] under a CC-BY license)
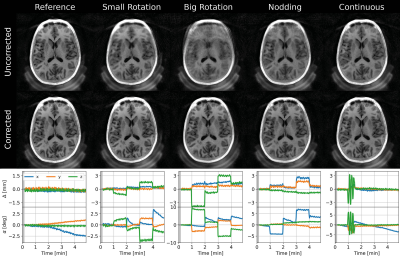
Figure 3: Representative axial slices with and without
motion correction for each motion paradigm from
subject 3, together with motion traces. Gray regions in the traces indicate
time for dummy segments and WASPI acquisition. (Reproduced with permission from
Ljungberg et al. 2022 [4] under a CC-BY
license)
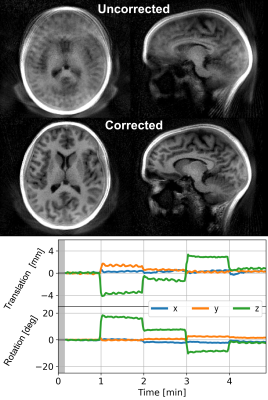
Figure 4: Example of the big rotation motion in subject 2 with rotation up to 20 deg.
(Adapted with permission from Ljungberg et al. 2022 [4] under a CC-BY license)
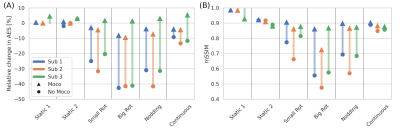
Figure 5: Quantitative
assessment of improvement in image quality after
motion correction using (A) Average Edge Strength (AES) and (B)
mean Structural Similarity Index Measure (mSSIM), both compared to the first
non-motion acquisition (“Static 1”). (Reproduced
with permission from Ljungberg et al. 2022 [4] under a CC-BY license)
DOI: https://doi.org/10.58530/2022/0002