0988
Self-gated 3D Stack-of-Spirals Ultra-Short Echo-Time Pulmonary imaging at 0.55T1Cardiovascular Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 2Siemens Medical Solutions USA Inc., Malvern, PA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD, United States, 5Department of Radiology and Imaging Sciences, Clinical Center, Department of Health and Human Services, National Institutes of Health, Bethesda, MD, United States
Synopsis
High-performance 0.55T MRI is promising for lung imaging due to the reduced susceptibility artifacts. However, high-resolution lung imaging is still challenged by low proton density and SNR. 3D spiral acquisitions can be used to improve SNR-efficiency, but these readouts are susceptible to trajectory errors and blurring from concomitant-fields, which are amplified at lower field strengths. Here we present a self-gated, ultra-short echo-time, stack-of-spirals acquisition which leverages rapid inline corrections for trajectory imperfections, trajectory dependent navigator signal fluctuations, and concomitant-fields to enable robust 1.75mm isotropic lung imaging at 0.55T. We also demonstrate our technique in healthy-volunteers, patients with lung-nodules and COVID-19.
Introduction
High-performance low field systems are well suited for structural lung imaging due to reduced susceptibility and prolonged T2* times1. Ultra-short echo time (UTE) sequences are important for high-resolution structural imaging of lungs at clinical field strengths2,3 but require dedicated optimization for low-field systems. SNR is reduced at lower-fields because it is proportional to field-strength. This can be mitigated by leveraging increased T2* times to efficiently sample k-space using longer spiral-readouts to improve SNR-efficiency. However, spiral-readouts are susceptible to off-resonance and concomitant-field related blurring. Off-resonance blurring scales with B0 and is therefore reduced at lower field strengths; however, concomitant field artifacts scale inversely with B0 and are amplified at lower field strengths4.Our aim is to develop a robust method for free-breathing, high-resolution pulmonary imaging at 0.55T. In this work, we optimized a self-gated stack-of-spirals UTE sequence and leverage trajectory correction, robust respiratory binning, and concomitant field correction to mitigate artifacts, with corrections implemented in line for rapid image reconstruction5.
Methods
Data Acquisition: A prototype free-breathing 3D T1-weighted UTE spoiled gradient echo stack-of-spirals sequence was used for imaging on a 0.55T MRI system (prototype MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). Additional readouts were acquired along the superior-inferior (SI) direction to estimate the respiratory-signal with temporal resolution of 200-300ms. All scans used golden angle increments of spiral interleaves. Imaging parameters: TE/TR = 0.5/7.7 ms, Flip-angle (FA) = 5, readout-duration = 5.4ms, field-of-view (FOV) = 450 x 450 x 224 mm3, matrix-size = 256 x 256 x 112 (slice oversampling-factor = 14.2%), total acquisition-time = 15 min 30 sec. We also retrospectively clipped the data from the end of the scan to simulate reduced scan times (5 min, 8.5 min, and 12 min) and its effect on image quality. We calculated relative maximum derivative to measure image sharpness around the diaphragm3.Reconstruction: Respiratory waveforms were extracted from the SI readouts using previously published methods6,7. An additional correction for trajectory dependent fluctuations in the respiratory navigator-signal was implemented. Data was retrospectively binned before reconstruction to reduce respiratory motion artifacts. The threshold for binning was empirically chosen to be 40% of the total data at the most stable respiratory phase.
Images were reconstructed inline using Gadgetron5 on a system equipped with Dual Intel Xeon processors, 512 GB RAM, 3x Nvidia Quadro RTX 8000 GPUs. Density compensation weights were estimated using an iterative technique8. We used a conjugate gradient SENSE reconstruction to reduce artifacts from non-uniform under-sampling in k-space for binned free-breathing data.
Spiral trajectory and concomitant field corrections: Inaccuracies in spiral trajectories were corrected using gradient system impulse response functions9,10. The local phase caused by concomitant fields was estimated for each spiral acquisition. Corrections were applied using multi-frequency interpolation, whereby each spiral acquisition was demodulated at 24-29 frequencies and a pixel-wise linear combination of the images was used to estimate the corrected image11.
Patient imaging: Human subjects imaging was performed with permission from the local Institutional Review Board. Informed consent was obtained from all subjects. A total of 15 human subjects were scanned for this study; 6 healthy volunteers, 3 patients with active COVID-19 infection, 4 patients who recovered from COVID-19, and 2 patients with lung nodules. The image quality and artifacts were qualitatively evaluated by a senior radiologist with 15 years of experience. Images were scored using a 5-level Likert scale for image quality and for artifact level.
Results
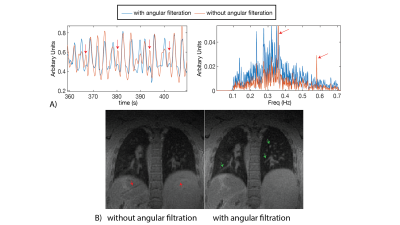
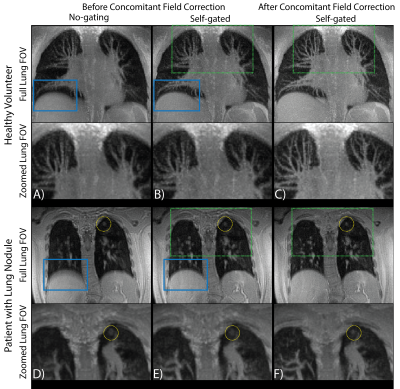
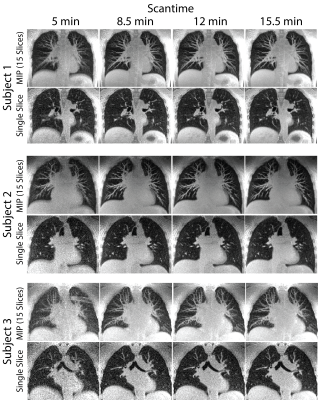
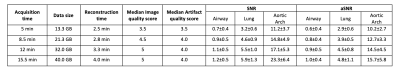
All reconstructions were performed in Gadgetron with reconstruction times < 5 min. Figure 1A shows an example of the self-gated respiratory signal with and without angular filtration. The resulting images (Figure 1B) demonstrate reduced respiratory motion artifacts especially noticeable around the liver dome and blood vessels with angular filtration. Improvements in image quality from respiratory binning and concomitant field correction are demonstrated in Figure 2 for a healthy volunteer and patient with a lung nodule.Figure 3 shows images reconstructed for scan times of 5, 8.5, 12, and 15.5 min in three subjects. Overall, we did not observe significant deterioration in image quality (i.e. significant loss in signal, or reduced visibility of anatomy) with simulated reduction in scan-time down to 8.5 min which shows that, potentially, an 8.5 min scan is sufficient to achieve diagnostic quality images with our technique. We also show the measured reduction in SNR and aSNR, and radiologist image quality score from shorter scan times in Table 1.
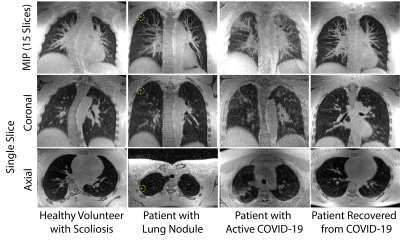
Figure 4 shows representative examples of maximum intensity projections (MIPs) and single-slice images from each subject group reconstructed from the 8.5 min acquisition. It includes a healthy volunteer, a patient with lung nodules, a patient with acute COVID-19 infection, and a patient who had recovered following COVID-19 infection.
Discussion and Conclusion
We have presented an optimized implementation of free-breathing 3D stack-of-spirals UTE pulmonary imaging sequence for a high-performance 0.55T system with fast inline reconstruction. We demonstrate that a combination of robust respiratory binning, trajectory corrections, and concomitant field corrections are necessary to achieve diagnostic image quality in high-resolution UTE imaging at 0.55T. Using our optimized method, we were able to achieve high-quality structural lung imaging with a resolution of isotropic 1.75mm in 8.5 min.Acknowledgements
The authors thank Margaret (Peg) Lowery, Jennifer Henry, Amelia Nargozian, and Doris Swaim for assistance with patient recruitment. The authors would like to thank Josef Pfeuffer for helpful discussions and assistance in design and implementation of the MR pulse-sequence used in this study. The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing cooperative research agreement (CRADA) between National Heart, Lung, and Blood Institute and Siemens Healthcare. The study was supported by the Division of Intramural Research, NIH/NHLBI (Z01-HL006257).References
- Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 2019;293:384–393.
- Delacoste J, Chaptinel J, Beigelman-Aubry C, Piccini D, Sauty A, Stuber M. A double echo ultra short echo time (UTE) acquisition for respiratory motion-suppressed high resolution imaging of the lung. Magn. Reson. Med. 2018;79:2297–2305.
- Zhu X, Chan M, Lustig M, Johnson KM, Larson PEZ. Iterative motion-compensation reconstruction ultra-short TE (iMoCo UTE) for high-resolution free-breathing pulmonary MRI. Magn. Reson. Med. 2020;83:1208–1221.
- King KF, Ganin A, Zhou XJ, Bernstein MA. Concomitant gradient field effects in spiral scans. Magn. Reson. Med. 1999;41:103–112.
- Hansen MS, Sørensen TS. Gadgetron: An open source framework for medical image reconstruction. Magn. Reson. Med. 2013;69:1768–1776.
- Sopra L Di, Piccini D, Coppo S, Stuber M, Yerly J. An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magn. Reson. Med. 2019;82:2118–2132.
- Zhang T, Cheng JY, Chen Y, Nishimura DG, Pauly JM, Vasanawala SS. Robust self-navigated body MRI using dense coil arrays. Magn. Reson. Med. 2016;76:197–205.
- Pipe JG, Menon P. Sampling density compensation in MRI: Rationale and an iterative numerical solution. Magn. Reson. Med. 1999;41:179–186.
- Campbell-Washburn AE, Xue H, Lederman RJ, Faranesh AZ, Hansen MS. Real-time distortion correction of spiral and echo planar images using the gradient system impulse response function. Magn. Reson. Med. 2016;75:2278–2285.
- Vannesjo SJ, Graedel NN, Kasper L, et al. Image reconstruction using a gradient impulse response model for trajectory prediction. Magn. Reson. Med. 2016;76:45–58.
- Man LC, Pauly JM, Macovski A. Multifrequency interpolation for fast off-resonance correction. Magn. Reson. Med. 1997;37:785–792.
Figures