S81
Optimizing a Motion Tracking Marker for Pediatric Patients1Radiology, Boston Children's Hospital, Boston, MA, United States, 2Computational Radiology Laboratory, Boston Children's Hospital, Boston, MA, United States
Synopsis
Magnetic Resonance Imaging (MRI) can be challenging for pediatric patients due to the large tunnel and loud noises. Anxiety and fear can be sparked causing them to be uncooperative and unable to hold still [1,2]. These exams are often plagued with motion artifacts resulting in poor diagnostic image quality and a challenge for Radiologists to interpret [3]. The administration of sedation or anesthesia is costly, potentially harmful, and doesn't eliminate motion artifacts due to breathing and uncontrollable muscle spasms [4]. There is an unmet need for alternative methods to enable diagnostic imaging exams in the presence of motion.
Background
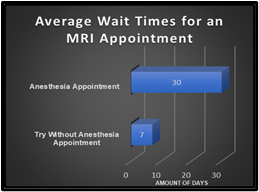
MRI is an important tool utilized in establishing a diagnosis and can influence treatment [3]. With imaging sequences tailored to the patient’s disease or symptoms, it is imperative to collect diagnostic images for interpretation. Motion artifacts can hinder a diagnosis and are costly to facilities. Now with COVID-19 precautions, facilities have required COVID-19 screenings for sedation and anesthesia appointments. These practices can cause further delays in scheduling and performing an exam. At our pediatric institution, the average wait time for an MRI exam with anesthesia is 30 days due to COVID-19 testing and practices (Fig. 1). There are also risk factors of administering sedation and anesthesia to pediatric patients such as “hypotension, myocardial depression, respiratory depression, hypertension, and hallucinations” [5]. In a previous study performed over the course of one year at the University of Michigan Health Care Systems, 22% of pediatric patients experienced an adverse reaction from sedation administered for an MRI or CT exam [6]. “The FDA has for the first time required a warning be added to the labels of general anesthetic and sedative medication to indicate the possible link between exposure to these drugs and adverse neurocognitive outcomes” [7]. The study of these medications and what long term affects they can pose to the neurocognitive development of pediatric patients is ongoing and needs to be further investigated, but current evidence supports an effort to use as low as reasonably achievable criteria for anesthesia and has identified no adverse long-term outcomes for procedures of similar length to a typical MRI [8,9].Method
To support the institution’s goal of significantly reducing MRI exams performed with sedation and anesthesia, our Radiology department purchased KinetiCor®, a motion tracking system, which employed with motion correction sequences, can help produce prospective motion corrected images. It was installed on a Siemens 3T Prisma MR scanner (Siemens, Erlangen, Germany) and involves attaching an adhesive disposable double winged motion tracking marker on the patient’s face. When the KinetiCor® system arrived, these double winged markers were not suitable for our pediatric population. The marker consisted of two wings each with a small square plate which were read by the internal infrared camera in the bore of the scanner. Each wing was securely fixed on the patient’s nose bridge with an adhesive backing (Fig.2). For our pediatric patients ranging in age from newborn to adult this posed two challenges. First newborns and infants have smaller facial structures and lack a ridged nose bridge. If the marker were placed on a newborn it would cover most of the face and would not be seen by the camera [10]. Second, we employ a video goggle system as a distraction technique which immerses the patient into their favorite movie. The goggles are consistently utilized for all age groups and with this nose bridge marker could not be applied. It was decided to create a pediatric friendly marker which consisted of one flat square plate on an adhesive band that could be placed on the forehead. As demonstrated in figure 3, the marker was securely away from the eyes and allowed the use of video goggles. Along with a KinetiCor® system our Radiology department offers a try-without anesthesia program to pediatric patients and their families which enables the patient to attempt their MRI exam with the support of a child life specialist rather than pharmaceutical support. With the pediatric friendly marker, we employed the KinetiCor® motion tracking system during these try-without appointments with the goal of achieving diagnostic prospective motion corrected images.Results
Currently we have utilized prospective motion correction sequences with the assistance of the Kineticor® system during a clinical exam for 55 patients. Each was consented with an appropriate IRB protocol from our institution. Out of these 55 patients, 7 were unable to acquire motion corrected sequences due to various circumstances such as technical difficulties and patient related challenges. Each were imaged with the single flat marker and ranged in age from 18 days to 21 years. We perform most brain exams with a 64-channel head coil which can interrupt the line of sight for the in-bore infrared camera. The anterior portion of the coil has bars which can block or cast shadows on the marker making it difficult for the infrared camera to locate the marker. Without this location, we are unable to perform the prospective motion correction sequences (Fig.2).Conclusion
Due to the need for diagnostic MRI without motion artifact, there is a demand for further advancements such as patient friendly motion correction techniques. Implementing a motion tracking system and prospective motion correction sequences can help support the goal of our institution to reduce sedation and anesthesia during imaging exams. For our pediatric population, optimizing the double winged marker to a single flat forehead marker has been beneficial. “This marker also allows for the most challenging patient population, 3 to 7 years of age, to be offered video goggles during appointments” [10]. By applying some creativity, we have been able to offer this technology to a wide population of ages from newborn to adult with the objective of achieving diagnostic images without sedation or anesthesia.Acknowledgements
No acknowledgement found.References
1. Robertson, R. L. et al. (2018) ‘Imaging Optimization in Children’, Journal of the American College of Radiology, 15(3), pp. 440–443.
2. Afacan, O. et al. (2016) ‘Evaluation of motion and its effect on brain magnetic resonance image quality in children’, Pediatric Radiology, 46(12), pp. 1728–1735. doi: 10.1007/s00247-016-3677-9
3. William Hollingworth, Christopher J. Todd, Matthew I. Bell, Qais Arafat, Simon Girling, Kantio R. Karia, Adrian K. Nixon, The Diagnostic and Therapeutic Impact of MRI: an Observational Multi-centre Study, Clinical Radiology, Volume 55, Issue 11, 2000, 825-831
4. Vanderby SA, Babyn PS, Carter MW, Jewell SM, McKeever PD. Effect of anesthesia and sedation on pediatric MR imaging patient flow. Radiology. 2010 Jul;256(1):229–237. PMID: 20505061
5. Camilo Jaimes & Michael S. Gee (2016) ‘Strategies to minimize sedation in pediatric body magnetic resonance imaging’, Pediatric Radiology, 46, pp. 916–927. doi: 10.1007/s00247-016-3613-z.
6. Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell DT, Wong JH, Tait AR. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth. 2000 Jun;84(6):743-8. doi: 10.1093/oxfordjournals.bja.a013586. PMID: 10895749; 745.
7. Callahan MJ, MacDougall RD, Bixby SD, Voss SD, Robertson RL, Cravero JP. Ionizing radiation from computed tomography versus anesthesia for magnetic resonance imaging in infants and children: patient safety considerations. Pediatr Radiol. 2018 Jan;48(1):21-30. doi: 10.1007/s00247-017-4023-6. Epub 2017 Nov 27. Erratum in: Pediatr Radiol. 2018 Jan 25;: PMID: 29181580.
8. Vinson AE, Houck CS. Neurotoxicity of Anesthesia in Children: Prevention and Treatment. Curr Treat Options Neurol. 2018 Oct 13;20(12):51. PMID: 30315440
9. Barton K, Nickerson JP, Higgins T, Williams RK. Pediatric anesthesia and neurotoxicity: what the radiologist needs to know. Pediatr Radiol. 2018 Jan;48(1):31–36. PMID: 28470388
10. Camilo Jaimes, M.D., Onur Afacan, Ph.D., Tess Wallace, Ph.D., Kristina Pelkola, RT (R)(MR), Lisa Chuah, Ph.D., Jenna Weldon, RT (R)(MR), Simon K. Warfield, Ph.D., Richard L. Robertson, M.D. (2019) ‘Prospective Motion Correction in Pediatric Neuroimaging with KinetiCor’, MAGNETOM Flash, 75, pp. 2–7
Figures