S74
Optimization of Radial K-space Sampling Techniques for a Comprehensive Motion-free Cervical Spine MRI Protocol1Philips Healthcare, Gainesville, FL, United States
Synopsis
Consistent high-quality magnetic resonance imaging (MRI) of the cervical spine still remains challenging because of the inherent small anatomical structures and degradation of image quality due to motion artifacts1. Motion artifacts can arise from several sources including swallowing, respiration, cerebrospinal fluid (CSF) pulsation, blood flow, and bulk patient movement. MRI of the cervical spine is one the highest performed exams globally. Here we present a complete cervical spine MRI protocol utilizing 2D and 3D radial k-space sampling techniques which are inherently motion insensitive to increase image quality.
Background
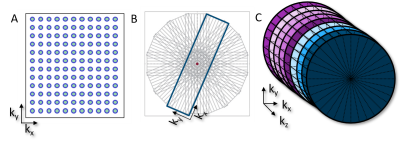
Consistent high-quality magnetic resonance imaging (MRI) of the cervical spine still remains challenging because of the inherent small anatomical structures and degradation of image quality due to motion artifacts1. Motion artifacts can arise from several sources including: swallowing, respiration, cerebrospinal fluid (CSF) pulsation, blood flow, and bulk patient movement2. MRI of the cervical spine is one the highest performed exams globally3. Therefore, optimization of cervical spine MRI protocols to improve IQ by reducing motion artifacts can have a significant clinical impact and increase diagnostic confidence. Radial k-space filling techniques such as PROPLLER (GE), BLADE (Siemens), and MultiVane (Philips) help to reduce motion artifacts (figure 1) by oversampling the center of k-space and can help improve image quality in the cervical spine1 (figure 2). In addition, a radial k-space trajectory helps to improve signal-to-noise (SNR) by the repeated oversampling of the center of k-space4 which can help to better visualize the smaller structures of the cervical spine. While use of sagittal T2-weighted radial acquisitions of the cervical spine have been shown to be advantageous in reducing motion artifacts1 this only represents a single sequence and contrast in a comprehensive MRI cervical spine exam. The American College of Radiology (ACR) guidance for MRI of the cervical spine includes acquiring multiple image contrasts in multiple imaging planes as well as utilization of 3D acquisitions to visualize small structures5. The ACR also recommends the use of motion suppression techniques where possible5. Here we present a complete MRI cervical spine protocol using both 2D and 3D radial k-space filling techniques to provide all necessary contrasts while reducing motion artifacts.Teaching Point
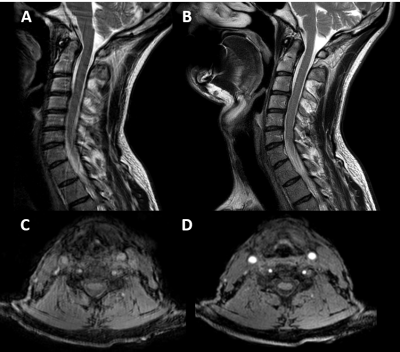
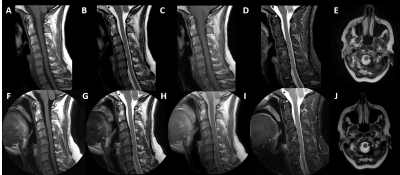
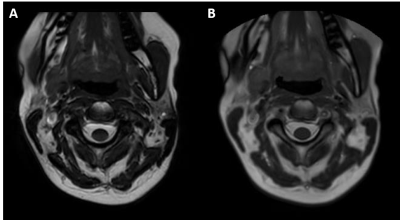
Current commercial 2D radial imaging offerings allow for the acquisition of turbo spin echo (TSE/FSE) and inversion recovery (IR) sequences to provide T1-weighted, T2-weighted, proton density, fluid attenuated inversion recovery (FLAIR), and diffusion weighted imaging (DWI) contrasts. Fat saturation techniques can also be applied to T2-weighted radial acquisitions to provide water sensitive images that meet ACR requirements. However, fat saturated T2-weighted images may fail compared to short tau inversion recovery (STIR) sequences over large a large field of view, in the presence of metal implants, and variations in B0 homogeneity. B0 homogeneity is drastically affected by all the motion in the cervical spine (i.e. swallowing, respiration, CSF and blood flow, etc.) which will lead to incomplete fat saturation based on spectral techniques. Simple modification of the inversion time (TI), echo time (TE), repetition time (TR), and echo train length (ETL) of the 2D radial FLAIR can be done to produce STIR images (Figure 3). Therefore, current commercially available 2D radial techniques (PROPELLER, BLADE, and MultiVane) can provide all necessary contrasts in the sagittal and axial plane. Advancements in radial techniques now allow for commercially available 3D stack of stars sequences (figure 1) to be acquired with and without fat saturation6. These techniques include StarVIBE (Siemens) and 3D VANE (Philips) and are most often used for T1-weighted free-breathing abdominal imaging. These sequences can be modified for cervical spine imaging (figure 3) especially in cases when the use of gadolinium-based contrast agents (GBCAs) are indicated. While axial 2D T2 and T2*-weighted images can be currently acquired, acquisition of a 3D radial T2*-weighted sequence has remained a missing element to provide a full compliment of radial image contrasts. Modification of the current 3D VANE implementation can be done to create a 3D T2*-weighted balanced fast field echo (bFFE) image of the cervical spine (figure 4).Summary
By simple modification and optimization of imaging parameters, it is possible to create a comprehensive motion-compensated cervical spine MRI protocol with all image contrasts. With the current ability to apply parallel imaging techniques (i.e. SENSE) to 2D and 3D radial acquisitions it makes the possibility of using a completely radial approach to reduce motion artifacts in the cervical spine clinically feasible. Overall protocol and scan times may be longer with radial acquisitions compared to Cartesian k-space trajectories with the same resolution, but even if one sequence needs to be repeated due to motion the comprehensive radial approach is faster (Table 1). Furthermore, the ability to acquire 3D T2*-weighted images is novel and provides an unmet clinical need to allow for the adoption of a completely motion compensated cervical MRI protocol. This approach can be applied at every field strength providing even higher applicability given that motion artifacts are more pronounced at 3T compared to 1.5T7. Based on the prevalence of MRI spine exams and the degradation of image quality from motion induced artifacts, adoption of a complete radial approach to imaging of the cervical spine can increase overall IQ, enhance workflow, and diagnostic confidence. Furthermore, with the development of newer acceleration techniques like compressed sensing8 and deep learning based reconstruction9 the ability to speed up radial scanning and implement this type of comprehensive motion-compensated imaging strategy across all exams may become more clinically feasible.Acknowledgements
No acknowledgement found.References
1. Fellner C, Menzel C, Fellner F, et al. BLADE in sagittal T2-weighted MR imaging of the cervical spine. American journal of neuroradiology. 2010;31(4):674-681.
2. Taber KH, Herrick RC, Weathers SW, Kumar AJ, Schomer DF, Hayman LA. Pitfalls and artifacts encountered in clinical MR imaging of the spine. Radiographics. 1998;18(6):1499-1521.
3. Anand PK, Shin DR, Saxena N, Memon ML. Accelerated Reliability Growth Test for Magnetic Resonance Imaging System Using Time-of-Flight Three-Dimensional Pulse Sequence. Diagnostics. 2019;9(4):164.
4. Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free‐breathing cardiac imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 1999;42(5):963-969.
5. Radiology ACo. ACR-ASNR-SBCT-MR Practice Parameter for the Performance of Magnetic Resonance Imaging (MRI) of the Adult Spine. Res19-2012, Amended. 2014.
6. Chandarana H, Block TK, Rosenkrantz AB, et al. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Investigative radiology. 2011;46(10):648-653.
7. Soher BJ, Dale BM, Merkle EM. A review of MR physics: 3T versus 1.5 T. Magnetic resonance imaging clinics of North America. 2007;15(3):277-290.
8. Lustig M, Donoho DL, Santos JM, Pauly JM. Compressed sensing MRI. IEEE signal processing magazine. 2008;25(2):72-82.
9. Lebel RM. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. arXiv preprint arXiv:200806559. 2020.
Figures