S19
Optimizing dark blood late gadolinium enhancement in cardiac magnetic resonance imaging.1MRC, Imperial College, London, United Kingdom
Synopsis
The dark blood late gadolinium enhancement technique has shown improved delineation of sub-endocardial infarcts by nulling both the blood pool and the normal myocardium. Optimal image quality and consistency depends on a number of factors, including gadolinium dose and the delay time before imaging. In this study we sought to determine whether gadolinium dose and delay time before imaging, affected the nulling of the blood pool and myocardium.
Background
Cardiac MRI provides vital diagnostic information to help clinicians decide if myocardial segments are viable after an ischemic event (1). Late gadolinium enhancement (LGE) imaging is the gold standard for detecting myocardial infarcts, which involve the sub-endocardium. However, due to the proximity of the blood pool, these infarcts can be easily missed on traditional LGE imaging techniques, which null the signal from healthy myocardium but not the blood pool. (2).Recently a novel single-shot motion-corrected balanced Steady State Free Procession (bSSFP) dark blood late gadolinium enhancement (DB-LGE) technique has shown improved delineation of sub-endocardial infarcts, by nulling both the blood pool and the normal myocardium (2). DB-LGE is achieved by using an additional T2 preparation pulse between the inversion recovery pulse and the readout (2). The optimum sequence timing is achieved by the user inputting the T1 values of healthy myocardium and blood pool, which have been obtained from a Modified Look-Locker Inversion recovery (MOLLI) T1 map performed immediately prior to the DB-LGE sequence (3).
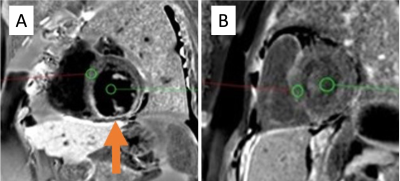
In clinical practice it was noted that the nulling of the blood pool signal in the DB-LGE imaging was more variable when there was a greater dose of gadolinium administered, despite employing the appropriate T1 values. This resulted in some DB-LGE images having poor blood pool nulling (Figure 1). It was also observed that those patients who had a longer delay between the gadolinium administration and image acquisition, exhibited better nulling of the blood pool signal.
Purpose
A recent change in local practice allowed investigation into these effects in patients who had received 0.1Mmols/kg of gadolinium and those who had received 0.15Mmols/kg.The hypothesis for this study was that a lower dose of gadolinium and a greater delay between gadolinium administration and image acquisition, results in more consistent nulling of the blood pool.
Method
This retrospective cohort study included patients who were imaged with the DB-LGE sequence in our unit between October and December 2020. All imaging was performed on a 1.5T Siemens Aera scanner using a 60-channel cardiac coil.All patients received Gadovist (Gadobutrol), either 0.1Mmol/kg or 0.15Mmol/kg depending on whether they were imaged before or after our change of practice. To determine the effectiveness of the nulling of the blood pool on the DB-LGE image, a ratio of the signal intensity of the blood pool and myocardium was measured. 6mm circular regions of interest (ROI) were placed in the blood pool, avoiding papillary muscles, and in healthy myocardium on a mid-short axis slice (Figure 1). Identical ROIs were placed on the T1 map to determine the T1 values used for the DB-LGE sequence.
An unpaired t test was performed in order to identify if the gadolinium dose influenced the nulling of the blood pool and myocardium. A linear regression was performed to investigate the relationship between the blood/myocardium ratio and the time elapsed from the injection of the gadolinium.
Results
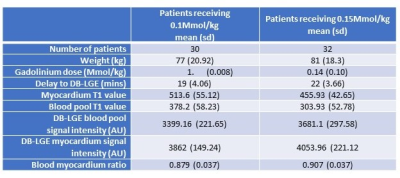
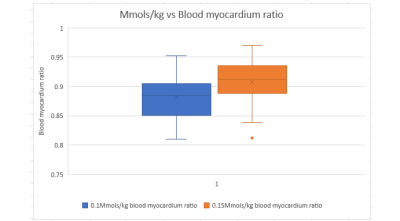
Images from 62 patients, 30 who received 0.1Mmols/kg and 32 who received 0.15Mmols/kg, were included in this study. Patient demographics and results are summarized in Figure 2.There was a statistically significant difference in the blood pool/myocardium ratio between those patients receiving 0.1Mmol/kg (0.879) and 0.15Mmol/kg (0.907) p>0.05. (Figure 3).
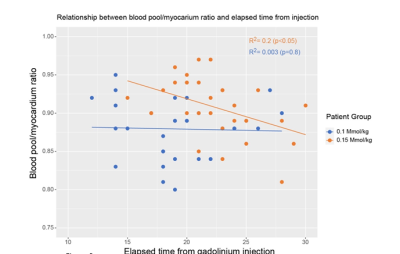
For patients who received 0.15 Mmol/kg of gadolinium there was a weak but statistically significant fit of the linear regression model for the relationship between the blood/myocardium ratio and the time elapsed from the injection of the gadolinium (R2=0.2, p<0.05). For patients who received 0.1Mmol/kg of gadolinium there was no such relationship (R2=0.003, p=0.8)(Figure 4).
Discussion
We have demonstrated that the change to a lower gadolinium dose has improved the blood pool nulling in the DB-LGE images, a finding supported by a previous study (2). This improvement in image contrast may mean that reporting clinicians have an increased confidence in identifying areas of infarct. Additionally, we showed that patients receiving the lower dose of gadolinium did not require as long a delay to achieve good blood pool nulling, which can benefit imaging departments by reducing scan times. This also leads to better cost efficiency as less contrast is required.Gadolinium deposition in the brain has been shown to occur in a dose dependent manner (4) with further evidence demonstrating brain and bone deposition occurring from both linear and macrocyclic contrast agents (5), therefore any reduction in gadolinium dose can be seen as beneficial to the patient. The retrospective nature of this study means that there were more extraneous variables we were unable to control, such as age and gender matching between the groups, which would have improved reliability and reproducibility.
Acknowledgements
No acknowledgement found.References
1. MRI in acute myocardial infarction (2011). M, Perazzolo Marra, J, Lima. S, Iliceto. European Heart Journal. https://doi.org/10.1093/eurheartj/ehq409
2. Dark blood late enhancement imaging (2016). Journal of Cardiovascular Magnetic Resonance. P, Kellman. H, Xue. L, Olivieri. R, Cross. E, Grant. M, Fontana. M, Ugander. J, Moon. M, Hansen.
3. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart (2004). Magnetic Resonance in Medicine. Messroghli, DR. Radjenovic, A. Kozerke, S. Higgins, DM. Sivananthan, MU. Ridgway, JP.
4. Gadolinium Deposition in Neurology clinical practice. (2019). The Ochsner Journal. Tyler, S. Steven, A. Bagart,B.
5. Macrocyclic and Other Non-Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue: Preliminary Results From 9 Patients With Normal Renal Function. (2016). Investigative Radiology. Murata, N. Gonzalez, L. Murata, K. Fligner, C. Dills, R. Hippe, D. Maravilla, K.
Figures