S11
Non-gated and non-enhanced MR Angiography of the Hand using enhanced acceleration selective arterial spin labeling (eAccASL)1radiological technology, Tokai university hospital, Isehara, Japan, 2Tokai University School of Medicine, Isehara, Japan, 3Philips Japan, Tokyo, Japan
Synopsis
This study was conducted to optimize the enhanced Acceleration-Selective Arterial Spin Labeling (eAccASL) for non-gated hand MRA. The MRA images obtained using four acceleration encoding (AENC) were assessed by visual evaluation for arterial depiction and venous contamination. The blood flow velocity obtained by phase-contrast cine was compared with the visual evaluation. It was found that the smaller the AENC (i.e. stronger motion sensitize gradients), the higher venous contamination and higher arterial visualization. The tendency of high visual evaluation was found in subjects with fast blood flow. The AENC of 0.5 or 1.0 was optimized for non-gated MRA of the hand.
Introduction
Since anti-angiogenic drugs can effectively alleviate symptoms in patients with rheumatoid arthritis, detecting abnormal synovial vessels is important for the diagnosis and treatment of rheumatoid arthritis.1 It has been reported that non-enhanced MRA of the hand using flow-sensitive dephasing prepared sequence was useful for the detection of abnormal synovial vessels.2 However, this MRA technique requires electrocardiographic gating, which leads to a long scan time and vulnerability for arrhythmia. Recently, a novel intracranial MRA using MSG (motion sensitization gradients) with motion compensation design was proposed, which enables enhanced acceleration sensitive spin labeling (eAccASL).3, 4 Therefore, we applied eAccASL technique to obtain MRA of the hand for without ECG gating. The objective of this study was to optimize the eAccASL for non-gated and non-enhanced MRA of the hand.Methods
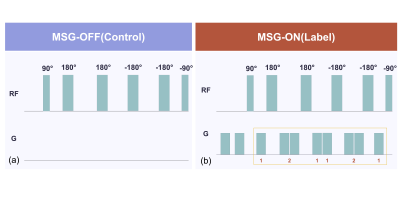
Twenty healthy volunteers were examined on a 1.5-Tesla MR system (Ingenia; Philips Healthcare, the Netherlands). The eAccASL technique is generated by subtracting the labeling image (only vein image) from the control image (artery and vein image) (Figure.1)3,4. We implemented eAccASL MRA to improve the B0 inhomogeneity on the hand, which was used turbo spin echo (TSE) sequence instead of steady-state free precession sequence. We used three-dimensional (3D) TSE based eAccASL sequence with the following parameters: TR, 3000ms; TE, 75 ms; field of view, 200*270 mm2; TSE factor, 80; number of slice; 55, parallel imaging factor, 2.0; phase interval, 2600ms; voxel size, 0.89*1.06*2.0 mm3; scan time, 4min. To assess the influence of the amplitude of the MSG, defined as acceleration encoding (AENC), we compared four different AENC values (i.e., 0.2, 0.5, 1.0, 1.5) on vessel visualization. The three visual evaluation on each AENC value was performed by two radiologists. Distal arterial depiction for two proper palmar digital arteries in each finger was defined as the maximum score of ten points. Proximal arterial depiction (ie., deep palmar arch and superficial palmar arch) was evaluated using a four-point scale: 1= No rating, 2= Only one palmar arch was visualized, 3= Two arches were incompletely visualized, and 4= Two arches were completely visualized. The venous contamination was also determined as four-point scale: 1= No rating, 2= Evaluation difficult, 3= Evaluable, 4= No effect. Each qualitative scoring was assessed using Friedman test and a post-hoc test. To investigate the index for the determination of the optimal AENC value, the peak to peak blood flow velocity (Vpp: subtracting minimum blood flow velocity from maximum blood flow velocity) was measured on common palmar digital arteries by phase contrast cine MRI. We assessed the correlation between the Vpp and qualitative parameters. Each correlation was identified with Spearman's rank-order correlation coefficient.Results and discussion
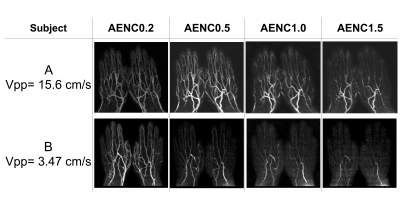
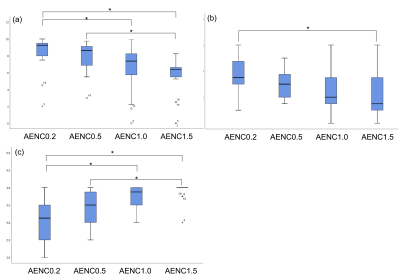
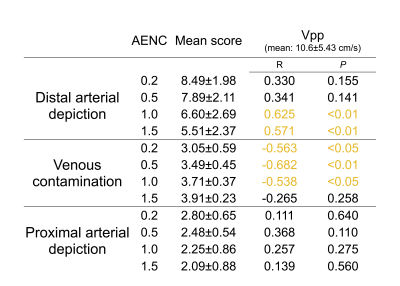
The quality differences were observed in the images of each subject (Figure.2). Although increasing AENC value (i.e. weak MSG) could suppress blood flow signals with smaller accelerations on subtracted images, some arteries were also suppressed in case of volunteers with low Vpp (bottom images). The mean and SD of Vpp was 10.6±5.43 cm/s (range, 1.76–20.93 cm/s) (Table.1). There were significant differences in both arterial depiction score and the venous contamination score among four AENC values (P < 0.01) (Figure.3). The distal arterial depiction on AENC value of 0.2 was significantly higher than that of 1.0 and 1.5 on the post-hoc analyses (P < 0.01). However, there was no significant difference between AENC value of 0.2 and that of 0.5. The proximal arterial depiction on AENC value of 0.2 was significantly higher than that of 1.5 (P < 0.01). On the other hand, the venous contamination score of AENC value of 1.5 demonstrated a high score. There were significant differences in the venous contamination between AENC value of 0.2 and that of 1.0 (P < 0.01), whereas there was no significant difference between AENC value of 0.5 and that of 1.0 (P < 0.01). These results indicate that AENC value of 0.5 or 1.0 was optimized for non-gated MRA of the hand. There were partial correlations between Vpp and distal arterial depiction and venous contamination (Table.1). On the other hand, proximal arterial depiction had no significant correlation. In subjects with large Vpp, almost arteries were often depicted at both AENC values of 0.2 and 1.5. Therefore, a significant difference was not observed for all AENC value. Since Vpp did not correlate with the proximal arterial depiction, it is considered that the arch has no velocity effect. In subjects with large Vpp, vein contamination appeared when the small AENC value was used. These results suggested that AENC value of 1.0 was more optimized than that of 0.5 in the case of the subject with large Vpp, and the optimum condition can be determined by measuring Vpp.Conclusion
The optimized eAccASL enables to provide non-gated MRA of the hand by determining the AENC value of 0.5 or 1.0.Acknowledgements
No acknowledgement found.References
(1) Koch AE et al. Arthritis&Rheumatism. 1998; 41(6): 951–962
(2) Li wang et al. Applied Magnetic Resonance. 2016; 47(8): 835-845
(3) Makoto Obara et al. Magnetic Resonance in Medicine. 2017; 77(5): 1996-2004
(4) Yuta Akamine et al. Magnetic Resonance in Medicine. 2019; 81(5): 3185-3191
Figures