Methods for Multiparameter Mapping
1High Field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 2Department of Biomedical Magnetic Resonance, University of Tübingen, Tübingen, Germany
Synopsis
This talk gives a technical overview about acquisition strategies suited to map longitudinal and transverse relaxation times simultaneously. Special focus is on fast joint T1 and T2 quantification based on three classes: multi-contrast steady-state free precession (SSFP) imaging, magnetization-prepared (MP) schemes with SSFP readout, and magnetic resonance fingerprinting (MRF) acquisitions. Possible acquisition strategies to enhance T2* sensitivity for simultaneous quantification of T1, T2, and T2* are introduced briefly as well.
Target audience
Researchers and clinicians interested in fast MR acquisition methods for simultaneous quantification of longitudinal and transverse relaxation times.Purpose
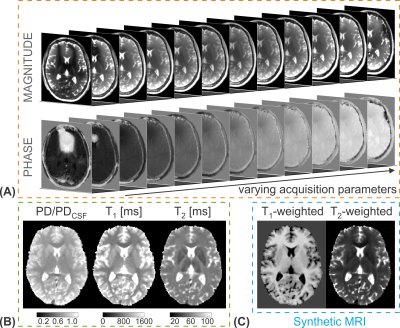
The rapid and accurate simultaneous quantification of multiple relaxation parameters based on the same acquisition scheme is a long-standing goal in the field of MR relaxometry. Multi-property techniques are often more efficient compared to sequential approaches and offer the calculation of intrinsically co-registered maps with identical motion state as well as matched chemical shift or distortion artifacts. Jointly quantifying multiple fundamental MR parameters such as relaxation times has gained further interest since a variety of typical clinically relevant weighted image contrasts can be synthesized from the derived maps in an approach known as synthetic MRI. Furthermore, the correlation between relaxation parameters in particular regions-of-interest can be investigated.Outline of educational talk
In the past years, various acquisition techniques have been presented to quantify longitudinal and transverse relaxation times simultaneously. We will consider joint T1 and T2 mapping methods based on three classes: multi-contrast steady-state free precession (SSFP) experiments, transient phase imaging after magnetization preparation with SSFP readouts, and magnetic resonance fingerprinting (MRF). The acquired signal evolution of many of these methods can be approximated well by analytical models (1,2), or numerical simulations using Bloch (3) or extended phase graph (EPG) (4) formalisms, facilitating the parameter estimation process. Recently, artificial neural networks have been proposed for simultaneous relaxation parameter prediction based on a voxelwise training process, taking measured or simulated multiparametric MR signal series as input (5-7).Multi-contrast SSFP sequences
Unspoiled short-repetition time SSFP sequences with balanced or nonbalanced acquisition schemes drive both longitudinal as well as transverse magnetization into a steady state. The generated signal exhibits an intrinsic mixed dependence on both T1 and T2, which can be utilized for multiparametric relaxation quantification. The possible SSFP acquisition schemes enabling simultaneous T1 and T2 relaxometry can be subdivided into two main groups:
- A series of phase-cycled balanced SSFP (bSSFP) scans allows sampling the characteristic tissue-specific frequency profile. T1 and T2 relaxation times can be estimated from the balanced SSFP profile by using motion-insensitive rapid configuration relaxometry (MIRACLE) (8) or an ellipse fitting approach termed PLANET (9).
- Multi-pathway nonbalanced SSFP
imaging offers the acquisition of several steady-state configurations including
higher order modes in a single scan. For simultaneous T1 and T2
estimation, at least the two lowest-order SSFP-FID
modes (F0, F1) and the lowest-order SSFP-Echo mode (F-1) need to be acquired
– an approach known as triple-echo steady-state (TESS) relaxometry (10). Enhanced T2*
weighting for concurrent T2* mapping can be introduced by sampling
the SSFP configurations at multiple echo times within a single scan (11,12).
Magnetization-prepared SSFP imaging
Magnetization-prepared (MP) techniques sample the transient signal recovery time course after an inversion pulse using rapid SSFP modules for readout. The recovery curves provide high T1 sensitivity while the SSFP readouts, e.g. balanced SSFP (as employed with IR-bSSFP (13-16)) or double-echo steady state (DESS) (as employed with MP-DESS (17)), introduce T2 sensitivity.
Magnetic resonance fingerprinting
MRF acquisitions in their original form (18) rely on the principle of IR-bSSFP while continuously varying specific parameters of the balanced SSFP readout in a pseudo-randomized manner throughout data collection, e.g., the repetition time or flip angle. Several hundred image contrasts are generated by highly undersampling the k-space, e.g., by the use of fast single-shot spiral trajectories. The second generation of MRF makes use of nonbalanced SSFP readouts (19) to avoid the off-resonance sensitivity of bSSFP. To add T2* sensitivity to the acquired signal evolution patterns, it has been proposed to quadratically vary the RF phases with balanced readouts (20) or to use variable echo times with nonbalanced schemes (21), enabling to quantify T1, T2, and T2* simultaneously.
Acknowledgements
No acknowledgement found.References
1. Zur Y, Wood ML, Neuringer LJ. Motion-insensitive, steady-state free precession imaging. Magn Reson Med 1990;16(3):444-459.
2. Hänicke W, Vogel HU. An analytical solution for the SSFP signal in MRI. Magn Reson Med 2003;49(4):771-775.
3. Bloch F. Nuclear Induction. Phys Rev 1946;70(7-8):460-474.
4. Weigel M. Extended phase graphs: dephasing, RF pulses, and echoes - pure and simple. J Magn Reson Imaging 2015;41(2):266-295.
5. Cheng CC, Preiswerk F, Madore B. Multi-pathway multi-echo acquisition and neural contrast translation to generate a variety of quantitative and qualitative image contrasts. Magn Reson Med 2020;83(6):2310-2321.
6. Heule R, Bause J, Pusterla O, Scheffler K. Multi-parametric artificial neural network fitting of phase-cycled balanced steady-state free precession data. Magn Reson Med 2020;84(6):2981-2993.
7. Cohen O, Zhu B, Rosen MS. MR fingerprinting Deep RecOnstruction NEtwork (DRONE). Magn Reson Med 2018;80(3):885-894.
8. Nguyen D, Bieri O. Motion-insensitive rapid configuration relaxometry. Magn Reson Med 2017;78(2):518-526.
9. Shcherbakova Y, van den Berg CAT, Moonen CTW, Bartels LW. PLANET: An ellipse fitting approach for simultaneous T1 and T2 mapping using phase-cycled balanced steady-state free precession. Magn Reson Med 2018;79(2):711-722.
10. Heule R, Ganter C, Bieri O. Triple echo steady-state (TESS) relaxometry. Magn Reson Med 2014;71(1):230-237.
11. Cheng CC, Preiswerk F, Hoge WS, Kuo TH, Madore B. Multipathway multi-echo (MPME) imaging: all main MR parameters mapped based on a single 3D scan. Magn Reson Med 2019;81(3):1699-1713.
12. Lee H, Wehrli FW. Alternating unbalanced SSFP for 3D R2' mapping of the human brain. Magn Reson Med 2021;85(5):2391-2402.
13. Schmitt P, Griswold MA, Jakob PM, Kotas M, Gulani V, Flentje M, Haase A. Inversion recovery TrueFISP: quantification of T1, T2, and spin density. Magn Reson Med 2004;51(4):661-667.
14. Ehses P, Seiberlich N, Ma D, Breuer FA, Jakob PM, Griswold MA, Gulani V. IR TrueFISP with a golden-ratio-based radial readout: fast quantification of T1, T2, and proton density. Magn Reson Med 2013;69(1):71-81.
15. Santini F, Kawel-Boehm N, Greiser A, Bremerich J, Bieri O. Simultaneous T1 and T2 quantification of the myocardium using cardiac balanced-SSFP inversion recovery with interleaved sampling acquisition (CABIRIA). Magn Reson Med 2015;74(2):365-371.
16. Bauman G, Pusterla O, Santini F, Bieri O. Dynamic and steady-state oxygen-dependent lung relaxometry using inversion recovery ultra-fast steady-state free precession imaging at 1.5 T. Magn Reson Med 2018;79(2):839-845.
17. Stöcker T, Keil F, Vahedipour K, Brenner D, Pracht E, Shah NJ. MR parameter quantification with magnetization-prepared double echo steady-state (MP-DESS). Magn Reson Med 2014;72(1):103-111.
18. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature 2013;495(7440):187-192.
19. Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med 2015;74(6):1621-1631.
20. Wang CY, Coppo S, Mehta BB, Seiberlich N, Yu X, Griswold MA. Magnetic resonance fingerprinting with quadratic RF phase for measurement of T2* simultaneously with δf, T1, and T2. Magn Reson Med 2019;81(3):1849-1862.
21. Wyatt CR, Smith TB, Sammi MK, Rooney WD, Guimaraes AR. Multi-parametric T2* magnetic resonance fingerprinting using variable echo times. NMR Biomed 2018;31(9).
Figures