4360
Altered cognition and emotion-related brain regions in asymptomatic carotid vulnerable plaque group: whole-brain voxel-wise analysis of IVIM1Department of Radiology, Xiangya Hospital, Central South University, Changsha, China, 2MR Research, GE Healthcare, Beijing, China
Synopsis
Altered cognition and emotion-related brain regions in patients with asymptomatic carotid vulnerable plaques : preliminary whole-brain voxel-wise analysis of intravoxel incoherent motion imaging
Objective
Carotid vulnerable plaques is regarded as a marker of higher ischemic or cognitive vulnerability of the brain, which may allow clinicians to identify subjects at risk of stroke and cognitive impairments and prompt an aggressive correction of cardiovascular risk factors, eventually including the initiation of treatment. Patients with carotid atherosclerosis with or without symptoms or increased carotid intima-media thickness often experience cognitive impairment and emotional symptoms (ESs) [1-3]. There are numerous classic functional MRI studies, including resting-state fMRI, diffusion tensor imaging (DTI) and artery spin labeling (ASL), to explore the changes of brain function and structure as diseases progress. In addition, a DWI-based IVIM in reflection of both microcapillary perfusion and tissue microstructure has been utilized to monitor tissue microvascular growth and degeneration [4]. Hence, we apply the bi-exponential model IVIM, which ascribes the signal attenuation in diffusion-weighted images to two main components, a slow one as water self-diffusion and a fast one as flow of water molecules in segments of the capillary network [5-6], to delineate voxel-wise differences of brain microstructure and microenvironment in patients with asymptomatic carotid vulnerable plaques compared to normal controls.Materials and Methods
Forty-nine elderly participants underwent IVIM DWI with a single-shot diffusion-weighted spin-echo echo-planar sequence using 20 different b-values (TR = 5600 ms; TE = 105 ms; imaging matrices= 256 x 256; slice thickness = 4 mm; axial slices = 28; gap = 5 mm; and 2 excitations, b values = 0, 10, 30, 50, 80, 100, 120, 150, 200, 300, 500, 800, 1000, 1300, 1500, 1800, 2400, 3000, 3600, and 4500 s/mm2) on 3.0 T MRI (Signa HDxt, GE Healthcare). Twenty-four participants with asymptomatic carotid vulnerable plaques and < 50% stenosis were served as the experimental group and the remaining as the healthy control group. The whole-brain slow ADC (Ds) and the fraction of fast ADC (f) values were voxel-wise compared with family-wise error (FWE) corrected threshold of P < 0.05.Results
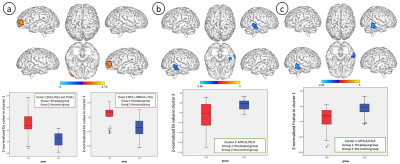
The Ds value and f values of five locations of the brain (ACA territory, MCA territory, PCA territory, anterior limb of internal capsule, posterior limb of internal capsule) between the ipsilateral hemisphere of unilateral carotid vulnerable plaques and the contralateral hemisphere were not significantly different (p > 0.05) (Table 1). Based on whole-brain voxel-wise comparison, for patients with carotid vulnerable plaques but < 50% stenosis, the Z-normalized Ds values were significantly higher than those of the control subjects in the left median cingulate and paracingulate gyrus (DCG.L), posterior cingulate gyrus (PCG.L), left precuneus gyrus (PCUN.L) (cluster size = 156), left middle frontal gyrus (MFG.L), orbital middle frontal gyrus (ORBmid. L) and superior frontal gyrus (SFG.L) (cluster size = 165). The Z- normalized Ds were significantly lower in the right middle temporal (MTG.R) and inferior temporal gyrus (ITG.R) (cluster size = 116), and the Z-normalized f values were significantly lower in the MTG.R and ITG.R (cluster size = 85) (p<0.05, FEW correction).Discussion
Several brain regions, especially DCG.L, PCG.L, MFG.L, SFG.L, ITG.R and MTG.R, had significantly higher (first four regions) and lower (latter two regions) Z-normalized Ds in the asymptomatic vulnerable carotid plaque group compared to the control group. The DCG, PCG and the precuneus are critical parts of the cognition and emotion-related default mode network (DMN) [7-9]. The left MFG and SFG play a role in stress meditation [10]. The SFG is also involved in cognitive functions and emotion regulation‐related processes, such as working memory and depression[11]. The anoxia-sensitive regions, ITG and MTG, are associated with word comprehension and anxiety [12-13]. Moreover, hypoperfusion in the ITG and MTG was found in patients with amnestic mild cognitive impairment (aMCI), indicating impaired function of blood oxygen supply [14]. In spite of brain lateralization in whole-brain voxel-based morphometry [15], normalized Ds and f maps in our study suggested that there was no lateralization in the changes in microcapillary perfusion and brain structure in patients with carotid vulnerable plaques possibly due to exclusion of patients with non-stenosed carotid artery whose circle of Willis still maintains cerebral hemodynamic changes.Conclusion
Our study was the first application of whole-brain voxel-wise comparisons of IVIM-derived normalized Ds and f values to show physiological alterations in cognition and emotion-related brain of patients with asymptomatic carotid vulnerable plaque.Acknowledgements
No acknowledgement found.References
[1] R. Everts, M. Wapp, Y. Burren, F. Kellner-Weldon, M. El-Koussy, K. Jann, et al.: Cognitive and emotional effects of carotid stenosis. Swiss Med Wkly, 144, w13970 (2014) doi:10.4414/smw.2014.13970
[2] C. T. Belem da Silva, M. S. Hoffmann, R. T. Sant Anna, F. C. Wehrmeister, H. Goncalves, I. O. Oliveira, et al.: Early Emotional Symptoms Predicting Carotid Atherosclerosis in Youth: Results From a Birth Cohort in Latin America. J Am Heart Assoc, 8(2), e011011 (2019) doi:10.1161/JAHA.118.011011
[3] J. Zhang, Z. Wang, M. Zhou, J. Jia, Y. Liu, A. Wang, et al.: Association Between Asymptomatic Vulnerable Carotid Plaques and Cognitive Impairment in Rural Adults. Front Neurol, 11, 662 (2020) doi:10.3389/fneur.2020.00662
[4] T. Finkenstaedt, M. Klarhoefer, C. Eberhardt, A. S. Becker, G. Andreisek, A. Boss, et al.: The IVIM signal in the healthy cerebral gray matter: A play of spherical and non-spherical components. NeuroImage, 152, 340-347 (2017) doi:10.1016/j.neuroimage.2017.03.004
[5] D. LeBihan,E.Breton,D.Lallemand,P.Grenier,E.CabanisandM.Laval-Jeantet: MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology, 161, 401-407 (1986) doi:10.1148/radiology.161.2.3763909
[6] D. Le Bihan, E. Breton, D. Lallemand, M. L. Aubin, J. Vignaud and M. Laval-Jeantet: Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology, 168, 497-505 (1988)
[7] R. Leech and D. J. Sharp: The role of the posterior cingulate cortex in cognition and disease. Brain, 137(Pt 1), 12-32 (2014) doi:10.1093/brain/awt162
[8] F. Caruana, M. Gerbella, P. Avanzini, F. Gozzo, V. Pelliccia, R. Mai, et al.: Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain, 141(10), 3035-3051 (2018) doi:10.1093/brain/awy219
[9] A. E. Cavanna and M. R. Trimble: The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564-83 (2006) doi:10.1093/brain/awl004
[10] S. Wang, Y. Zhao, L. Zhang, X. Wang, X. Wang, B. Cheng, et al.: Stress and the brain: Perceived stress mediates the impact of the superior frontal gyrus spontaneous activity on depressive symptoms in late adolescence. Hum Brain Mapp, 40(17), 4982- 4993 (2019) doi:10.1002/hbm.24752
[11] T. A. Niendam, A. R. Laird, K. L. Ray, Y. M. Dean, D. C. Glahn and C. S. Carter: Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci, 12(2), 241-68 (2012) doi:10.3758/s13415-011-0083-5
[12] L. Bonilha,A.E.Hillis,G.Hickok,D.B.denOuden,C.RordenandJ.Fridriksson: Temporal lobe networks supporting the comprehension of spoken words. Brain, 140(9), 2370-2380 (2017) doi:10.1093/brain/awx169
[13] M. Li, H. Xu and S. Lu: Neural Basis of Depression Related to a Dominant Right Hemisphere A Resting-State fMRI Study. Behavioural Neurology, 5024520 (2018) doi:10.1155/2018/5024520. eCollection 2018
[14] W. X, D. D, Z. Q, L. X, P. L, Z. X, et al.: Brain hemodynamic changes in amnestic mild cognitive impairment measured by pulsed arterial spin labeling. Aging (Albany NY), 12(5), 4348-4356 (2020) doi:10.18632/aging.102888. Epub 2020 Mar 12
[15] G. Gainotti: The influence of handedness on hemispheric representation of tools: a survey. Brain Cogn, 94, 10-6 (2015) doi:10.1016/j.bandc.2014.12.005
Figures