4355
Fast Tractography Demonstrates the Topography of the Corpus Callosum In Ideal and Post-stroke Conditions
Jacqueline Chen1, Mark Lowe1, Ken Sakaie1, Kenneth Baker2, Andre Machado3, and Stephen Jones1
1Cleveland Clinic Imaging Institute, Cleveland, OH, United States, 2Cleveland Clinic Lerner Research Institute, Cleveland, OH, United States, 3Cleveland Clinic Neurological Institute, Cleveland, OH, United States
1Cleveland Clinic Imaging Institute, Cleveland, OH, United States, 2Cleveland Clinic Lerner Research Institute, Cleveland, OH, United States, 3Cleveland Clinic Neurological Institute, Cleveland, OH, United States
Synopsis
Accurate white-matter tractography maps can be a useful clinical tool for assessing neurological disorders, however, incorrect assumptions within tractography algorithms can yield non-physiological results. We have developed a tractography methodology based on probability theory that uses both local and global information to improve accuracy, and standard partial differential equation solvers for fast whole-brain mapping. In this abstract we demonstrate: 1) the accuracy of the method by comparing a topographical map of the corpus callosum (CC) generated from a symmetrized human data phantom to published maps; 2) how differences in CC topography may be associated with stroke location and functional disability.
Introduction
Fast, whole-brain maps of white-matter (WM) tracts can serve as a useful clinical tool for assessing structurally intact or damaged pathways present in patients suffering from neurological diseases, including: stroke, multiple sclerosis and epilepsy. The use of diffusion MRI to estimate brain WM tracts has been recently reviewed,1 emphasizing the need to limit biased results and strive for biological accuracy. To address these concerns, we present a tractography methodology based on probability theory that uses both local and global information to improve accuracy, and standard partial differential equation solvers for fast whole-brain mapping. First, we demonstrate the accuracy of the method, by comparing a topographical map of the corpus callosum (CC) generated from a symmetrized human data phantom to published maps from pathological and diffusion tractography investigations. Second, we demonstrate how differences in CC topography may be associated with the location and functional disability of patients who suffered from stroke.Methods
The method is based on our previous work.2 Our probabilistic approach includes both local voxel information from the high angular diffusion imaging data, and the global conditions that require tracks to start at a seed and end at a target without intersecting a non-WM boundary.Demonstrating accuracy
A symmetrical phantom was computed from human data. Using our tractography method with the Freesurfer3 segmentation, the flux from right hemisphere cortex (RHC) to left hemisphere cortex (LHC) was calculated (Fig. 1), and seed voxels from 35 RHC regions were used for tracking to the LHC. A unified topographical CC map was defined based on transcallosal track clusters and compared to published maps.
Demonstrating differences in CC topography in relation to stroke location and functional disability
We analyzed 7 tesla anatomical and diffusion MRI data from 2 patients (Table). Patient A was imaged 30 months after a stroke in right frontal and parietal WM and exhibited dysfunction of their affected upper limb. Patient B was imaged 22 months after a stroke in the left putamen and exhibited better function in both affected and unaffected upper limbs compared to Patient A. Using our tractography method with the Freesurfer segmentation, the flux from RHC to LHC was calculated, and seed voxels from right precentral cortex were used for tracking to the LHC. Maps of the intersection of the transcallosal tracks with the CC were generated and compared.
Results
Demonstrating accuracy- Anterior CC (Fig. 2, A) contained tracks from the most anterior and inferior regions of prefrontal cortex, consistent with findings from diffusion tractography,4 pathology,5 and T1 relaxometry.6
- Mid-Anterior CC (Fig. 2, B) contained tracks mostly from superior and middle frontal cortical regions, consistent with findings from diffusion tractography.4, 7
- Central and Mid-Posterior CC (Fig. 2, C) contained tracks from cortical regions proximal to the central sulcus (precentral, paracentral, postcentral) and lateral sulcus (insula, transverse temporal, superior temporal, supramarginal), consistent with findings from pathology.5
- Posterior CC (Fig. 2, D) contained tracks from posterior parietal, inferior temporal and occipital lobes, consistent with findings from diffusion tractography,4, 7 and pathology.5
When considering the intersection of the transcallosal fibers connecting right and left precentral cortices with the CC, we found that Patient A’s fibers extended a shorter distance along the CC (Fig. 3, B) compared to Patient B (Fig. 3, C) and the symmetrized human data phantom (Fig. 3, A).
Discussion
We have developed a tractography methodology based on probability theory that uses both local and global information to improve accuracy, and standard partial differential equation solvers for fast whole-brain mapping. The speed of the method lends itself to clinical use, allowing for probing of multiple pathways to evaluate injury. To demonstrate accuracy of the method, we derived a topographical map of the CC generated from a symmetrized human data phantom, and compared it to published CC maps. Our method showed consistency with published findings from diffusion tractography, pathology and T1 relaxometry studies. To demonstrate the clinical relevance of mapping the transcallosal pathways, we evaluated 2 patients with chronic stroke injury. The patient with more pronounced upper-limb deficit exhibited a shorter extent of the CC occupied by transcallosal fibers connecting right and left precentral cortices compared to the patient with less upper-limb deficit and compared to the symmetrized human data phantom.Conclusion
Our fast WM tractography method generates topographical maps of the CC that are consistent with published findings from diffusion tractography, pathology, and T1 relaxometry studies. Analysis of clinical data suggests that quantification of transcallosal tracks may be an important metric of brain injury.Acknowledgements
No acknowledgement found.References
- Jeurissen B, Descoteaux M, Mori S, Leemans A. Diffusion MRI fiber tractography of the brain. NMR Biomed. 2019 Apr;32(4):e3785.
- Zhang M, Sakaie KE, Jones SE. Logical foundations and fast implementation of probabilistic tractography. IEEE Trans Med Imaging. 2013 Aug;32(8):1397-410.
- Laboratory for Computational Neuroimaging at the Athinoula A. Martinos Center for Biomedical Imaging. FreeSurfer. [cited 2020 18 Jun]; Available from: http://surfer.nmr.mgh.harvard.edu/.
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum Brain Mapp. 2008 May;29(5):503-16.
- Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: the history of the corpus callosum. Braz J Med Biol Res. 2003 Apr;36(4):409-20.
- Lee BY, Zhu XH, Li X, Chen W. High-resolution imaging of distinct human corpus callosum microstructure and topography of structural connectivity to cortices at high field. Brain Struct Funct. 2019 Mar;224(2):949-60.
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006 Sep;32(3):989-94.
Figures
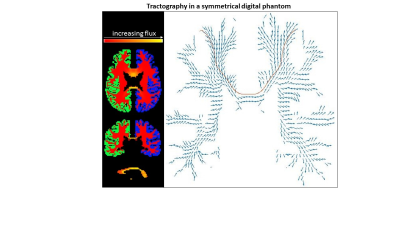
Figure 1: (Left) The
flux magnitude (red-yellow) from right (green) to left (blue) cortices of the
symmetrized human data phantom. (Right) Flux vectors in x- and z- directions (blue arrows). Brown line is a single
track from a single seed voxel.
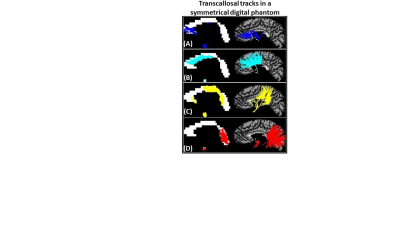
Figure 2: (A) Anterior CC contained tracks from the most anterior and
inferior regions of prefrontal cortex. (B) Mid-Anterior CC contained tracks from caudal
anterior cingulate and
superior & middle frontal cortical regions.
(C) Central
and Mid-Posterior CC contained tracks from cortical regions proximal to
the central sulcus (precentral, paracentral, postcentral) and lateral sulcus
(insula, transverse temporal, superior temporal, supramarginal). (D) Posterior
CC contained tracks from posterior
parietal, inferior temporal and occipital lobes.
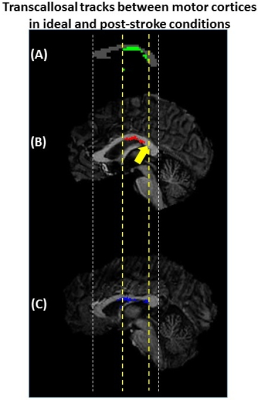
Figure 3: (A) Phantom mid-sagittal CC showing the ideal
intersection of the transcallosal tracks (green) between precentral gyri. (B) The intersection of tracks (red)
between precentral gyri for Patient A shows a region where no
tracks were detected (yellow arrow) but were found in (A). (C) The intersection
of tracks (blue) between precentral gyri for Patient B had a similar
extent along the CC as the phantom.
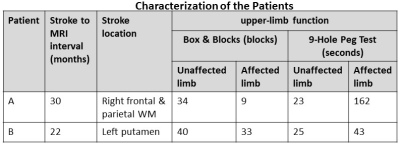
Table: The patients were chosen to differ based on upper limb
dysfunction. The Box & Blocks Test measures unilateral gross manual
dexterity. The score is the number of blocks that were successfully manipulated
in 1 minute. The 9-Hole Peg Test measures finger dexterity. The score is the
time required to complete the task. The average score from 2 trials is shown.