Greg O Cron1, Rafael Glikstein2, Jean Francois Thibodeau3, Anthony Carter3, Chet E Holterman3, Alexey Gutsol3, Lihua Zhu3, Baptiste Lacoste3, and Chris Kennedy3
1Ottawa Hospital Research Institute, Ottawa, ON, Canada, 2The Ottawa Hospital, Ottawa, ON, Canada, 3University of Ottawa, Ottawa, ON, Canada
Synopsis
For hypertensive patients taking low-dose aspirin, there may be risk of kidney injury. NSAIDs block vasoactive prostaglandin production, potentially blunting the reopening of hypertension-constricted renal vessels, thereby decreasing renal blood flow. We hypothesized that hypertension would predispose rats to low-dose aspirin induced kidney and cerebrovascular injury. Hypertensive rats who received low-dose daily aspirin appeared to have decreased cerebral blood flow, however the data must be interpreted with caution, as statistical power was lacking. Of interest, rats given both AngII and ASA developed significant kidney and cerebrovascular injury, suggesting a possible deleterious effect of this drug combination on the vasculature.
Introduction
Patients taking low-dose aspirin to reduce the probability of future heart attack or stroke are usually well-informed about the risk of major bleeding. For hypertensive patients, however, nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin may carry an additional risk of kidney injury. NSAIDs block vasoactive prostaglandin production, potentially blunting the reopening of hypertension-constricted renal vessels, thereby decreasing renal blood flow (1). We recently showed evidence of this in rodent models of hypertension (2-4). In this study, we hypothesized that hypertension would predispose rats to low-dose aspirin induced kidney and cerebrovascular injury, due to reduced blood flow.Methods
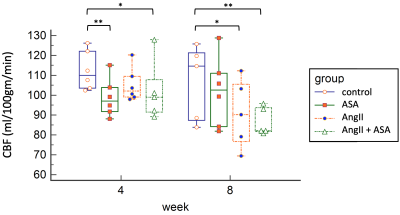
Four groups of animals were used: 1) control - wild-type male Sprague Dawley rats, n=6 ; 2) ASA - rats given low-dose acetylsalicylic acid (aspirin) at 1mg/kg/day, n=6; 3) AngII – rats given Angiotensin II at 200ng/kg/min to induce hypertension, n=6; 4) AngII+ASA – rats given the combination, n=7. The experiment was run for 8 weeks, with drugs delivered by osmotic minipumps, implanted subcutaneously at baseline. Whole-brain cerebral blood flow (CBF) was assessed in vivo using dynamic contrast-enhanced MRI at baseline, +4 and +8 weeks (5). Urinary albumin levels were measured by ELISA as a surrogate of kidney injury. At endpoint, cerebral and renal vascular injury were assessed by histology and immunohistochemistry for CD-31 and Caveolin-1.Results
Two rats from the AngII+ASA group died between weeks 0 and 4, leading to only n=5 at weeks 4 and 8 for that group. At +4 weeks, CBF for ASA and AngII+ASA were lower than controls (Dunnett’s multiple comparisons test, P=0.003 and 0.04). At +8 weeks, CBF for AngII and AngII+ASA were lower than controls (P=0.03 and 0.004). Unique to the AngII+ASA group, we observed cerebral vascular remodeling of pial, intracerebral arteries/ arterioles as well as thickened arterial media, narrowed lumens and accumulation of PAS-positive material. Caveolin-1 was significantly enhanced in the endothelium and the media of the intracerebral arteries, arterioles and capillaries. Assessment of kidney injury revealed thickening of the arterial media with increased accumulation of PAS-positive material, rarefaction of smooth muscle cell (SMC) nuclei, accumulation of monocytes in the adventitia. Caveolin-1 was significantly enhanced while CD31 was reduced in glomeruli, interlobular and arcuate arteries. Albuminuria was highest for AngII+ASA, however due to high variance this was not statistically significant.Conclusions
Hypertensive rats who received low-dose daily aspirin appeared to have decreased CBF, however the data must be interpreted with caution, as statistical power was lacking. Of interest, rats given both AngII and ASA developed significant kidney and cerebrovascular injury, suggesting a possible deleterious effect of this drug combination on the vasculature.Acknowledgements
No acknowledgement found.References
1. Whelton A. Am J Ther. 2000 Mar;7(2):63-74. 2. Cron et al, Proc. ISMRM 23 (2015), abstract # 1575. 3. Thibodeau, Jean-Francois, et al. Antioxidants and Redox Signaling (2016). 4. Cron et al, Proc. ISMRM 26 (2018), abstract # 4598. 5. Livingston, Jessica M., et al. Neurobiology of Disease 137 (2020): 104756.