4344
Central pulse pressure and its influence on carotid artery morphology predict white matter hyperintensity volumes1Cardiff University Brain Research Imaging Centre (CUBRIC), School of Physics and Astronomy, Cardiff University, Cardiff, United Kingdom, 2CUBRIC, School of Medicine, Cardiff University, Cardiff, United Kingdom, 3Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 4CUBRIC, School of Psychology, Cardiff University, Cardiff, United Kingdom, 5Cardiff Metropolitan University, Cardiff, United Arab Emirates, 6Cardiff Metropolitan University, Cardiff, United Kingdom
Synopsis
Cardiovascular risk factors have been linked with deteriorations in cerebrovascular function in the brain, causing cell loss, particularly in the white matter. The mechanisms by which cardiovascular risk factors influence the microvasculature is unclear. Increased blood pressure and associated arterial stiffness/morphological changes in larger cerebral vessels are thought to play a key role. Here, we demonstrate that central pulse pressure along with associated changes in carotid radius and tortuosity better predict the white matter lesion burden in the brain compared with other measures of blood pressure and systemic arterial stiffness.
Introduction
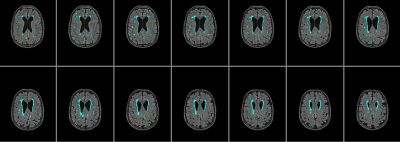
White matter hyperintensities (WMHs) are small regions of demyelination and axonal loss observed in the deep white matter structures of the brain, which are thought to be caused by ischaemic damage in small vessels, and appear on T2-weighted FLAIR MRI as regions of hyperintense signal. These lesions are commonly seen in older subjects, and associated with stroke and dementia, particularly vascular dementia1, and with arterial stiffness2 . Due to these associations, such lesions are thought to be influenced by cardiovascular disease such as hypertension or atherosclerosis. Hypertension is a risk factor, normally diagnosed with a brachial measure of blood pressure. However, this measure may not be as important as central pulse pressure (CPP) in the development of WMHs. The Strong Heart Study showed CPP to be more strongly related to carotid hypertrophy, development of atherosclerotic plaques, and incidence of stroke than brachial pressure in a group of over 3000 adults3, and central systolic pressure has also been shown to be associated with carotid atherosclerosis and WMH2. Brachial pulse pressure (BPP) is higher than carotid pulse pressure in young adults4, and a greater increase in CPP than BPP with age has been documented. Measurement of CPP and its effect on the morphology of the carotid artery may aid in determining the risk of WMHs. Arterial stiffness, hypertrophy and increased tortuosity are known to occur in hypertension and atherosclerosis5, and so measuring CPP in conjunction with imaging of carotid artery structure and white matter hyperintensities may reveal a relationship between cardiovascular and arterial factors in the development of WMH.Methods
Data was collected on 42 participants, with an age range of 55-84, and a BMI range 19-33. 3 participants did not complete all scans, one participant was excluded due to inconsistencies in CPP measurement. 24 female participants and 11 male participants were included in the analysis (mean age = 67 years). CPP was derived from carotid-femoral arterial stiffness measurements (Pulse Wave Analysis) in the supine position using the SphygmoCor (AtCor Medical, Australia) system. A single high-fidelity tonometer was used. Participants were supine for 30 minutes while blood pressure measurements were taken from the wrist, neck, and top of the leg. Participants underwent MRI scanning at 3T (Siemens 3T Prisma) at Cardiff University Brain research Imaging Centre (CUBRIC). Participants were fasted for a minimum of 4 hours and abstained from caffeine, alcohol and intense exercise for 12 hours preceding the scanning session. A T2-weighted FLAIR scan, a T1-weighted MPRAGE scan, and a time-of-flight (TOF) angiography scan were performed. FLAIR and MPRAGE images were processed using a pre-trained deep learning algorithm6 to create masks of WMHs. The Vascular Modeling Toolkit (VMTK)7 was used to extract 3D images of the internal carotid arteries from TOF scans, and to calculate the tortuosity and average radius for each artery. Measurements from the two arteries were then averaged. Linear regression was performed in Matlab to identify correlations between BP, CPP, carotid tortuosity, carotid radius and WMH volume.Results
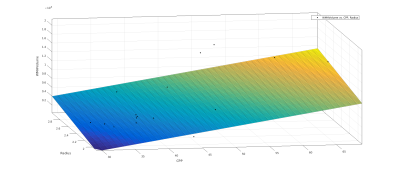
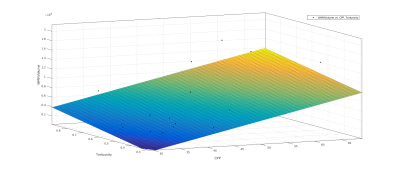
Linear regression showed a correlation between systolic BP and total WMH volume (R-squared = 0.152, P = 0.033), but not between diastolic BP and total WMH volume (R-squared = 0.0451, P = 0.26). This correlation was improved by a multiple linear regression comparing both systolic and diastolic BP with WMH volume (R-squared = 0.243, P = 0.0232). Pulse wave velocity as a standard measure of arterial stiffness showed no significant correlations with WMH volume (R-squared = 0.0509, P = 0.2). CPP showed a stronger correlation with WMH volume (R-squared = 0.29, P = 0.00122). While carotid radius or tortuosity alone showed little correlation with WMH volume (radius R-squared = 0.0665, P = 0.147, tortuosity (R-squared = 0.0247, P = 0.382), a multiple linear regression showed a significant correlation between combined CPP and radius with WMH volume (R-squared = 0.311, P = 0.00375) and between combined CPP and tortuosity with WMH volume (R-squared = 0.319, P = 0.00316).Discussion
We shows that brachial blood pressure and pulse wave velocity are poor indicators of risk of white matter hyperintensities. Carotid pulse pressure may be a more reliable indicator of risk of WMH, and measures of arterial structure can give further insight into the relationship. The influence of CPP on the morphology of the carotid artery may be the key predictor of underlying risk for vascular dysfunction that causes WMHs.This is a work in progress and further investigation of the influence of CPP on both large and small cerebral vessels is warranted. Extending the analysis to the morphology of the middle cerebral artery and use of white matter maps to target smaller branching arteries may provide insight into the relationship between local vascular structure and white matter hyperintensities. A more nuanced measure of WMHs accounting for the FLAIR signal intensity may give us further insight into the relationship between vascular factors and WMHs. Combining this analysis with PCASL scans will reveal the dysfunction caused at the microvascular level.
Acknowledgements
The authors thank the Wellcome Trust for funding this project (funding ref: WT200804)References
1) Wardaw JM, Valdés Hernández MC, Muñoz-Maniega S, What are white matter hyperintensities made of? J Am Heart Assoc 2015; 4: e001140
2) Shrestha I, Takahashi T, Nomura E, Ohtsuki T, Ohshita T, Ueno H, Kohriyama T, Matsumoto M. Association between central systolic blood pressure, white matter lesions in cerebral MRI and carotid atherosclerosis. Hypertens Res 2009; 32: 869-874
3) Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure. Hypertension 2007; 50: 197-203
4) McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: current evidence and clinical importance. Eur Heart J 2014; 35: 1719-1725
5) Schriffin EL. Vascular remodeling in hypertension. Hypertension 2012; 59: 367-374
6) Li H, Jiang G, Zhang J, Wang R, Wang Z, Zheng W, Menze B. Fully convolutional network ensembles for white matter hyperintensities segmentation in MR images. Neuroimage 2018; 183: 650-665.
7) Antiga L, Piccinelli M, Botti L, Ene-Iordache B, Remuzzi A and Steinman DA. An image-based modeling framework for patient-specific computational hemodynamics. Medical and Biological Engineering and Computing 2008; 46: 1097-1112
Figures