4337
Processing cerebrovascular reactivity data using shift-invariant dictionary learning1Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom, 2UK Dementia Research Institute, Edinburgh, United Kingdom, 3Institute for Digital Communications, University of Edinburgh, Edinburgh, United Kingdom
Synopsis
The BOLD response to a hypercapnic challenge, i.e. cerebrovascular reactivity (CVR), may vary between individuals and tissue types. Linear regression (GLM) between the BOLD signal and the end-tidal CO2 is the most common CVR processing method but does not allow for different haemodynamic responses across the brain. We propose to use shift-invariant dictionary learning (SIDL) as a promising method to enable data-driven extraction of BOLD response(s). We show that CVR and delay estimates from SIDL are comparable to estimates from GLM. Future work will focus on reducing the effect of drift on SIDL estimates.
Introduction
Cerebrovascular reactivity (CVR) measures the ability of blood vessels to dilate in response to demand for energy in the brain. CVR is often investigated in diseases associated with cerebrovascular dysfunction such as small vessel disease (SVD)1. CVR data are often acquired with blood-oxygen-level-dependent (BOLD) magnetic resonance imaging (MRI) during a hypercapnic challenge. CVR can be measured in vivo as the relative change in the BOLD signal divided by the change in end-tidal CO2 (EtCO2), most commonly by linearly regressing (general linear model, GLM) the BOLD signal to the EtCO22,3. However, there are several limitations to this method including assuming an instantaneous haemodynamic response to CO2 and that all tissues exhibit the same haemodynamic response. New processing methods are needed to address these issues.Shift-invariant dictionary learning (SIDL)4 has previously been used to detect ischemia in cardiac phase-resolved myocardial BOLD MRI5,6. It is a promising technique for measuring CVR as it can extract different haemodynamic responses from the data. We applied SIDL to calculate CVR magnitude and delay and compared it to the standard processing method.
Theory
A circulant dictionary is a circular matrix where each column is a down circular-shifted version of the first column known as the kernel. Let $$$Y\in \Re^{t×n}$$$ be the BOLD dataset where $$$n$$$ is the number of voxels and $$$t$$$ the number of MRI time points. SIDL, a dictionary learning algorithm, finds in a least squares sense, a circulant dictionary $$$C\in \Re^{t×p}$$$, with $$$p$$$ allowed shifts, and sparse representations $$$X\in \Re^{p×n}$$$ such that the dataset $$$Y≈CX$$$. The optimisation problem is: $$min_{C,X} ||Y-CX||_F$$ $$s.t. ||x_i||_0≤s, s\ll t, i=1,...,n \tag{1}$$ $$||c_j||_2=1, k=1,...,t$$where (sparsity) is the number of non-zero elements of the column $$$i$$$ of $$$X$$$, $$$c_j$$$ is the column $$$j$$$ of $$$C$$$ and $$$||·||_F$$$ designates the Frobenius norm. To solve eq. 1, SIDL alternately updates $$$X$$$ and $$$C$$$ using orthogonal matching pursuit7 and circulant dictionary learning algorithm4 respectively. Applying SIDL with $$$s=1$$$ on CVR data gives a coefficient $$$x_i$$$ associated to CVR for voxel $$$i$$$ and a CVR delay corresponding to the associated column $$$j$$$ of the dictionary.
On the other hand, GLM assumes a linear relationship between the BOLD time courses and the EtCO2. It consists in modelling the BOLD time course of a voxel as2: $$BOLD(t)≈β_0+β_1·t+β_2·EtCO2(t) \tag{2}$$
Methods
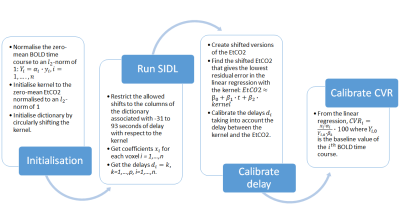
We acquired CVR data from 20 SVD patients (mean age: 64.5, females: 35%) using 2D gradient-echo echo-planar imaging (TR = 1550 ms, TE = 30 ms, FOV = 235×235×125 mm) on a 3T Siemens Prisma MRI scanner and a block-design paradigm (2-3-2-3-2 minutes alternating between medical air and 6% CO2-enriched air) with simultaneous measurement of EtCO28. We manually segmented the deep grey matter structures using an established procedure and ran SIDL and GLM on a voxel-wise basis in those regions. For the analysis, we also computed the mean BOLD signal as the mean BOLD signal across voxels in deep grey matter.The SIDL dictionary was initialised to the EtCO2. Details of the pipeline are shown in Figure 1. To compare CVR and delay values from GLM2 with CVR and delay values from SIDL, we scaled the SIDL CVR values to units of %/mmHg and delays relative to the EtCO2.
Results
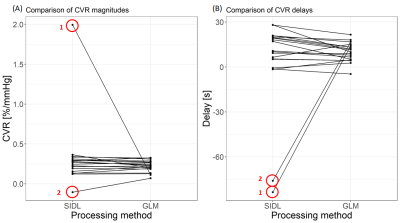
Figure 2 shows the comparison of CVR magnitudes and delays from SIDL and GLM. SIDL gives slightly higher CVR magnitude (mean difference: 0.10 %/mmHg) and lower delay (mean difference: -6.3 s) values, but were overall comparable to those given by the GLM. There were two extreme outliers for SIDL: both have a drift in the BOLD signals (Figure 3). Removing the outliers from the analysis gives a mean CVR difference of 0.01%/mmHg and mean delay difference of 3.6 s between the two processing methods.Discussion
We showed that SIDL with one dictionary gives CVR and delay estimates broadly comparable to GLM. CVR magnitudes obtained with SIDL were marginally higher supposedly because the shape of the kernel better approximates the BOLD time courses than the EtCO2. However, two outliers were present with SIDL. In those two subjects, the dictionary depicted signal components that were not apparent in the mean BOLD signal, including drift. Further optimisation is needed to apply SIDL to CVR analyses: drift could be regressed during pre-processing or an additional dictionary could be used to isolate drift.SIDL has the advantage of using a data-driven approach to obtain the regressor driving the CVR response, as opposed to assuming this to be the EtCO2 profile as in the GLM. Other methods, including the iterative principal component analysis method (e.g.RIPTiDe9), can also be used to extract a regressor from the data. Comparison between CVR estimates from SIDL and RIPTiDe will be investigated in future work. Additionally as eq. 1 can be extended to the use of multiple dictionaries (or haemodynamic responses) to represent the BOLD signals5 without prior segmentation, SIDL may enhance sensitivity to differences between tissues, potentially associated with impairment.
Conclusion
SIDL is a promising method for CVR data processing. We validated its use by comparing its CVR magnitude and delay estimates with those obtained using GLM. Future work will include optimisation to reduce the effect of drift and using additional dictionaries to account for differences in BOLD signal between tissues.Acknowledgements
This work was funded by the Medical Research Council (MRC) and UK Dementia Research Institute (UK DRI) which receives its funding from DRI Ltd, funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK, the European Union Horizon 2020, PHC-03-15, project No 666881 ‘SVDs@Target’, the Fondation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease, ref no. 16 CVD 05, and Scottish Chief Scientist Office through NHSLothian Research and Development Department. M.J.T. acknowledges financial support from the NHS Lothian Research and Development Office.
We thank Dr. Una Clancy and Daniela Garcia for their involvement in the recruitment of patients in the Mild Stroke Study 3. We also thank the participants, radiographers and professional support staff for their involvement in this work.
References
1. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696.
2. Thrippleton MJ, Shi Y, Blair G, et al. Cerebrovascular reactivity measurement in cerebral small vessel disease: Rationale and reproducibility of a protocol for MRI acquisition and image processing. Int J Stroke. 2018;13:195–206.
3. Liu P, De Vis JB, Lu H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. NeuroImage. 2019;187:104–115.
4. Rusu C, Dumitrescu B, Tsaftaris SA. Explicit Shift-Invariant Dictionary Learning. IEEE Signal Process Lett. 2014;21:6–9.
5. Rusu C, Morisi R, Boschetto D, Dharmakumar R, Tsaftaris SA. Synthetic Generation of Myocardial Blood–Oxygen-Level-Dependent MRI Time Series Via Structural Sparse Decomposition Modeling. IEEE Trans Med Imaging. 2014;33:1422–1433.
6. Rusu C, Tsaftaris SA. Structured Dictionaries for Ischemia Estimation in Cardiac BOLD MRI at Rest. In: Golland P, Hata N, Barillot C, Hornegger J, Howe R, editors. Med Image Comput Comput-Assist Interv – MICCAI 2014. Cham: Springer International Publishing; 2014. p. 562–569.
7. Pati YC, Rezaiifar R, Krishnaprasad PS. Orthogonal matching pursuit: recursive function approximation with applications to wavelet decomposition. Proc 27th Asilomar Conf Signals Syst Comput. 1993. p. 40–44 vol.1.
8. Clancy U, Garcia DJ, Stringer MS, et al. Rationale and design of a longitudinal study of cerebral small vessel diseases, clinical and imaging outcomes in patients presenting with mild ischaemic stroke: Mild Stroke Study 3. Eur Stroke J. SAGE Publications; Epub 2020 Jun 5.:2396987320929617.
9. Donahue M.J., Strother M.K., Lindsey K.P., Hocke L.M., Tong Y., Frederick B.D.B. Time delay processing of hypercapnic fMRI allows quantitative parameterization of cerebrovascular reactivity and blood flow delays. J Cereb Blood Flow Metab. 2016;36:1767–1779.
Figures