4336
Detecting Magnetic Resonance Changes in Brain Structure and Function During Stroke Rehabilitation1School of Biomedical Sciences and Pharmacy, University of Newcastle, Newcastle, Australia, 2School of Health Sciences, University of Newcastle, Newcastle, Australia, 3Hunter Medical Research Institute, Newcastle, Australia, 4Faculty of Health and Medicine, University of Newcastle, Newcastle, Australia, 5University of Newcastle, Newcastle, Australia
Synopsis
Using Magnetic Resonance (MR) data acquired as part of a feasibility study in stroke rehabilitation, a novel post-processing pipeline was designed and implemented to explore metabolic factors with MR Spectroscopy (MRS). The stroke study looked at the effect of aerobic exercise performed immediately prior to the usual task-specific rehabilitation training. Examining the clinical motor function results in relation to the metabolic data revealed by the MRS pipeline showed some interesting correlations among metabolites and rehabilitation outcomes.
BACKGROUND
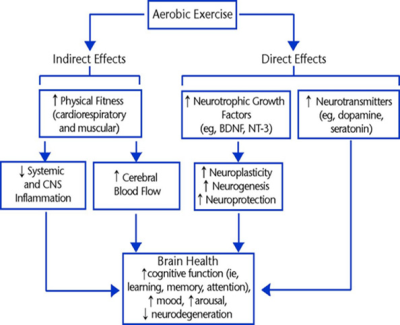
In stroke rehabilitation, the recovery of lost function is attributed to neuroplasticity; changes in neural structure and function which support adaptation that restores connectivity. Aerobic exercise has been shown to be a potent stimulator of neuroplastic brain chemistry[1-3]. MR techniques are frequently used to both diagnose and monitor stroke patients, offering images with high contrast and resolution [4, 5]. MR imaging (MRI) can provide anatomical reference for visual or computational lesion mapping, tissue segmentation, or acute diagnosis, while MRS offers data on the metabolic properties of the neural tissue[6]. Applying MRS in neuroplasticity studies enables non-invasive analysis of the neurochemistry in-vivo, to explore the metabolites involved in stroke recovery. The post-processing of MRS imaging (MRSI) data can become a substantial task at scale, as several (>30) metabolites are measured by MRS and multi-slice, multi-voxel scans are becoming the norm[7]. It has become evident that utilising MRS data in studies of any size, demands data post-processing pipeline software to accelerate the research analysis. This study was designed to develop a novel MRS post-processing pipeline and to then test its performance and accuracy. The output of the pipeline software would then be used in analysis of stroke rehabilitation clinical motor function data.MATERIALS AND METHODS
The MRS data was acquired during a feasibility study of aerobic exercise (AEX) as supplemental to the usual task-specific training in stroke rehabilitation[8, 9]. 10 of the total 20 stroke participants provided MR data at baseline and 9 of 10 provided follow up data at 12 weeks. The MR data was acquired using a 3T Siemens MRI to capture T1 and T2 weighted structural images as well as an MRSI sequence. The earlier study grouped participants into AEX+TST group (n=9) and TST (n=11) intervention groups. The MR data was acquired from a subgroup (n=9) comprising of AEX+TST (n=4) and TST (n=5). Clinical motor functions were assessed by common measurement standards. The pipeline (inSPECT) was written in Python 3 for its high-level features in succinct and functional code. A Graphical User Interface (GUI) formed the foundation of the design process. A popular data format for data exchange is Comma Separated Values (CSV). The CSV could be processed to include voxels and metabolites of interest to the study, and mark excluded values. Segmentation of partial brain tissue volumes was achieved with a novel stroke pipeline in MATLAB, adapted from one designed for multiple sclerosis[10], using FSL-FAST software; inSPECT had features added to apply partial volume fractions by a correction formula to the metabolite concentrations, per voxel, per scan data. Finally, inSPECT would compile the results conveniently grouped for statistical analysis. The MRSI data was then collated and analysed with clinical motor rehabilitation measures from the basis study.RESULTS
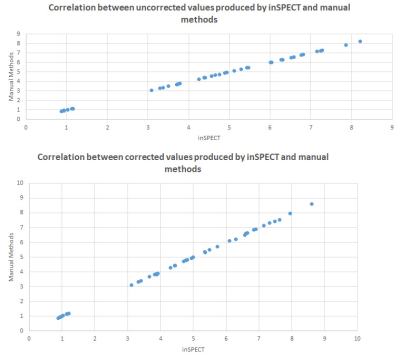
Cohen’s Kappa was calculated between automated pipeline results and manual processing for each metabolite concentration per voxel, participant, and time-point κ = 1.00. The correlation between the automated pipeline results and manual processing results was rS = 1.00 for both the uncorrected and segmentation-corrected data. Time to process data manually was 7m 20s raw filtering and 4m 45s segmentation correction, totalling 12m 5s per participant, per scan. inSPECT required < 1s for all data. Exploratory analysis of metabolite concentrations revealed several notable cases of significant and strong correlation including right hemispheric glutathione (GSH) and right hemispheric N-acetylaspartate (NAA) (r=0.905), right hemispheric glutamine+ glutamate (Glx) and right hemispheric NAA (r=0.900) and left hemispheric Glx and left hemispheric total creatine (tCr) (r=0.881) (all at baseline). Change from baseline to follow up correlations of note are, right hemispheric GSH and right hemispheric Glx (0.929), right and left hemispheric tCr and Wolfe Motor Function Test (WMFT) time (0.714 & 0.857 respectively), right hemispheric Glx and VO2peak (0.857) and left hemispheric GPC and Six Minute Walking Test (6MWT) (0.898). There were no significant within- or between- group changes in any of the clinical outcomes or key metabolite concentrations.DISCUSSION
The use of MRSI in research is gaining traction, however the challenge to process the data produced has limited its appeal. The new software pipeline “inSPECT”, has enabled the simple application of MRS analysis in this and future studies. It has performed the role of data selection and post-processing efficiently and accurately. In the context of this study, the MRS results have shown that there are potential links between motor cortex neuroplasticity and clinical measures of rehabilitation and recovery. The feasibility study which provided this data was however not designed with statistical power to reveal significance in metabolic results, and therefore the correlations should be viewed as a guide to future research hypotheses.CONCLUSION
The pipeline software was feasible to develop and effective in accurately, reliably, and promptly processing the MRS data from output by LCModel to input to statistical software. Analysis of metabolic and clinical motor assessments revealed interesting correlations that may link to neuroplastic responses to aerobic exercise, but low statistical power urges caution in interpretation of these findings.Acknowledgements
The author would like to thank the University of Newcastle and the Hunter Medical Research Institute, all the supervisors and other staff, and family and friends.References
1. Shafer, M., The Effects of Acute Bouts of Aerobic and Resistance Exercise on Neuroplasticity. 2020. 2. Constans, A., et al., Influence of Aerobic Training and Combinations of Interventions on Cognition and Neuroplasticity after Stroke. Front Aging Neurosci, 2016. 8: p. 164. 3. Murdoch, K., J.D. Buckley, and M.N. McDonnell, The effect of aerobic exercise on neuroplasticity within the motor cortex following stroke. PloS one, 2016. 11(3): p. e0152377. 4. Aben, H.P., et al., A Role for New Brain Magnetic Resonance Imaging Modalities in Daily Clinical Practice: Protocol of the Prediction of Cognitive Recovery After Stroke (PROCRAS) Study. JMIR Res Protoc, 2018. 7(5): p. e127. 5. Macintosh, B.J. and S.J. Graham, Magnetic resonance imaging to visualize stroke and characterize stroke recovery: a review. Frontiers in neurology, 2013. 4: p. 60-60. 6. Carlson, H.L., et al., Spectroscopic biomarkers of motor cortex developmental plasticity in hemiparetic children after perinatal stroke. Hum Brain Mapp, 2017. 38(3): p. 1574-1587. 7. Tremblay, S., et al., The use of magnetic resonance spectroscopy as a tool for the measurement of bi-hemispheric transcranial electric stimulation effects on primary motor cortex metabolism. J Vis Exp, 2014(93): p. e51631. 8. Valkenborghs, S.R., et al., Aerobic exercise and consecutive task-specific training (AExaCTT) for upper limb recovery after stroke: A randomized controlled pilot study. Physiother Res Int, 2019. 24(3): p. e1775. 9. Valkenborghs, S.R., et al., AExaCTT - Aerobic Exercise and Consecutive Task-specific Training for the upper limb after stroke: Protocol for a randomised controlled pilot study. Contemp Clin Trials Commun, 2017. 7: p. 179-185. 10. Quadrelli, S., C. Mountford, and S. Ramadan, Hitchhiker's guide to voxel segmentation for partial volume correction of in vivo magnetic resonance spectroscopy. Magnetic resonance insights, 2016. 9: p. MRI. S32903. 11. Oberlin, L.E., et al., Effects of physical activity on poststroke cognitive function: a meta-analysis of randomized controlled trials. Stroke, 2017. 48(11): p. 3093-3100.Figures
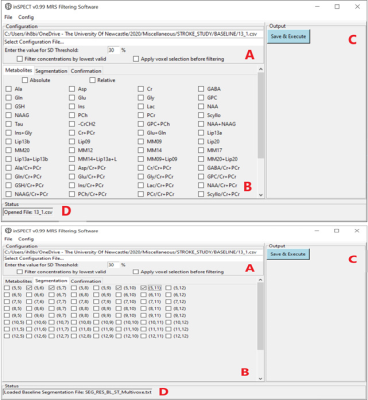
Figure 1i: inSPECT GUI displaying list of selectable metabolites (B), the configuration panel (A) Cramer-Rao lower bounds SD% threshold. Output panel (C) contains the button to execute processing of data, the lower edge (D) is the status bar, indicating a single CSV file has been opened.
Figure 1ii: inSPECT GUI with voxel selection tab active (B) with voxels checked for filtering. (A) is selected file information and checkboxes for inputting the concentration filter method. Panel (C) is the Save & Execute button, panel (D) is current status, a Baseline Segmentation file has been loaded.