4333
Impaired cerebrovascular reactivity in patients with Huntington's disease1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 3Department of Neurology, Massachusetts General Hospital, Boston, MA, United States
Synopsis
Multiple subcortical white matter areas showing impaired cerebrovascular reactivity (CVR) in our pre-symptomatic and early Huntington Disease (HD) subjects overlap with the white matter areas and tracts that have been reported to demonstrate altered microstructural integrity. They are also adjacent to cortical brain regions that atrophy as disease progresses in HD. Our findings support that abnormal cerebrovascular function contributes to the neuropathology of HD.
Introduction
There is increasing evidence that impairments of cerebrovascular function and/or abnormalities of the cerebral vasculature might contribute to early neuronal cell loss in patients with Huntington’s disease (HD). Previous studies have used an exogenous carbon dioxide (CO2) challenge in conjunction with functional magnetic resonance imaging (fMRI) to assess regional cerebrovascular reactivity (CVR) in healthy individuals as well as in patients with neurodegenerative disorders. In this study, we explored potential impairments of CVR in patients with HD.Subjects and Methods
Twenty-three participants were included. Eleven were healthy volunteers and the remaining 12 were gene-expanded individuals, including both pre-symptomatic and early HD. All MRI scanning was performed at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital. All the experimental procedures were explained to the subjects and signed informed consent was obtained prior to participation in the study. All procedures were approved by the Institutional Review Board at MGH.MRI brain scanning was performed on a 3-Tesla scanner (Siemens Medical, Erlangen, Germany) using a standard head coil. Acquisition parameters included: 1) standard high-resolution 3D sagittal images acquired with volumetric T1-weighted MEMPRAGE (TR=2530ms, TE=1.74ms/3.6ms/5.46ms/7.32ms, flip angle=7º, FOV=256mm, matrix=256×256, slice thickness=1mm); 2) T2-SPACE (TR=3200ms, TE=454, flip angle=120º, FOV=256×256mm, matrix=256×256, slice thickness=1mm); 3) BOLD-fMRI images acquired with gradient-echo echo planar imaging (EPI) sequence (TR=1250ms, TE=30ms, flip angle=90º, FOV=220×220mm, matrix=64×64, thickness=5mm, slice gap=1mm) during the hypercapnic challenge. Subjects wore a nose-clip and breathed through a mouth-piece using an MRI-compatible circuit designed to maintain the PETCO2 within ± 1-2 mmHg of target PETCO2 [1,2]. The fraction of inspired carbon dioxide was adjusted to produce steady-state conditions of normocapnia and mild hypercapnia (4-8 mmHg above the subject’s resting PETCO2). The CO2 challenge paradigm consisted of 2 consecutive phases (normocapnia and mild hypercapnia) repeating 6 times with 3 epochs of 4 mmHg increase and 3 epochs of 8 mmHg increase of PETCO2. The normocapnia phase lasted 60-90 seconds, while the mild hypercapnia phase lasted 30 seconds. The total duration of the exogenous CO2 challenge lasted 10 minutes.
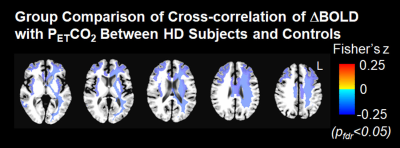
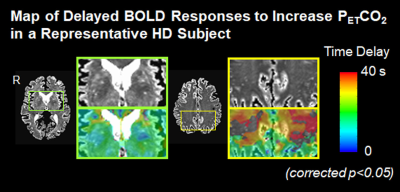
A Hilbert Transform analysis [3] was used to compute the cross-correlation between the time series of regional BOLD signal changes (ΔBOLD) and increased PETCO2, and to estimate the response delay of ΔBOLD relative to PETCO2. CVR impairment is indicated by the weak cross-correlation between the time series of regional ΔBOLD and increased PETCO2. Fisher’s Z-transformation was used to convert the cross-correlation coefficients in the individual subject maps to Fisher’s z scores for the group comparison between HD subjects and healthy controls. In addition, we evaluated the relationship between regional ΔBOLD and PETCO2 to perivascular load, as assessed by the volume of dilated perivascular spaces (PVS). The regional volume of dilated PVS in HD subjects was quantified using T1- and T2-images, and the detailed procedures were described in our previous study [4]. Increased PVS load has been shown recently in early symptomatic HD [4].
Results
After correcting for age, we found that the cross-correlation between the time series for regional ΔBOLD and for PETCO2 in several subcortical and deep white matter regions was significantly weaker in HD subjects than in controls (Figure 1). They included body of corpus callosum, subcortical white matter adjacent to rostral and caudal anterior cingulate, rostral and caudal middle frontal, insular, middle temporal, posterior cingulate areas, temporal poles, and deep white matter. In addition, greater dilated PVS load was observed along the periphery of the areas showing the greater ΔBOLD response delay (Figure 2), although this correlation was not significant.Discussion
Impaired CVR is predominantly found in subcortical white matter areas in HD. The subcortical white matter areas that showed weaker cross-correlation between the time series for regional ΔBOLD and for PETCO2 are adjacent to the cortical brain regions which have previously been reported to have atrophy in HD subjects [5]. Impaired CVR found in these white matter areas is also consistent with abnormal structural changes in white matter tracts of forceps major, superior longitudinal fasciculus and cingulum bundle previously reported in early HD subjects [6].Conclusion
Our findings support that alterations in cerebrovascular function occur in HD and the dominance of such alterations in white matter areas further suggests that HD has the signs of small vessel disease. The impaired cerebrovascular reactivity may be an important, not as yet considered, contributor to early neuropathology in HD.Acknowledgements
Support for this research was provided by the National Institutes of Health: R01NS106384 and the Dake Foundation. We are very grateful to the patients and families who so generously contributed to this work.References
1. Banzett RB, Garcia RT, Moosavi SH. Simple contrivance "clamps" end-tidal PCO(2) and PO(2) despite rapid changes in ventilation. J Appl Physiol 2000;88(5):1597-1600.
2. McKay LC, Evans KC, Frackowiak RS, Corfield DR. Neural correlates of voluntary breathing in humans. J Appl Physiol 2003;95(3):1170-1178.
3. Saad ZS, DeYoe EA, Ropella KM. Estimation of FMRI response delays. Neuroimage 2003;18(2):494-504.
4. Chan ST, Mercaldo ND, Ravina B, Hersch SM, Rosas HD. Association of dilated perivascular spaces and disease severity in patients with Huntington's disease. Neurology 2020.
5. Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, Greve D, Hevelone N, Hersch SM. Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity. Brain 2008;131(Pt 4):1057-1068.
6. Rosas HD, Wilkens P, Salat DH, Mercaldo ND, Vangel M, Yendiki AY, Hersch SM. Complex spatial and temporally defined myelin and axonal degeneration in Huntington disease. Neuroimage Clin 2018;20:236-242.
Figures