4326
Relationship of FLIAR vascular hyperintensity territory with APCVs and venous oxygen saturation in patients with cerebral infarction1The First Affiliated Hospital of Dalian Medical University, Da Lian, China, 2Philips Healthcare, Bei Jing, China
Synopsis
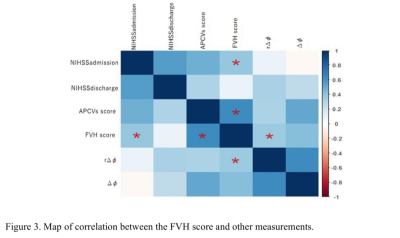
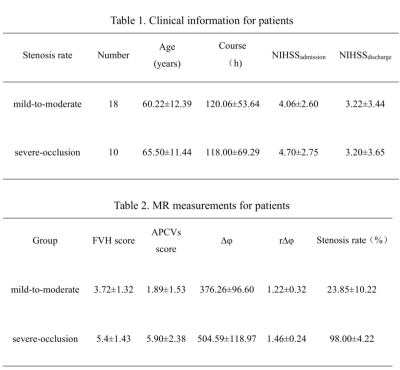
Previous studies have suggested that fluid-attenuated inversion recovery vascular hyperintensity (FVH) and asymmetrical prominent cortical veins (APCVs) may be related with collateral flow in cerebral ischemia. Some studies showed susceptibility-weighted imaging (SWI) can reflect oxygenation fraction (OEF) in venous blood. Here, we prospectively compared the differences of FVH and APCVs scores in different types of vascular stenosis, and analyzed the relationships of the FVH score with the APCVs score, the venous-oxygen-saturation related MR measurements as well as the NIHSS score in patients with cerebral infarction. It was found that FVH and APCVs scores in severe-occlusion group were significantly higher than those in mild-to-moderate group. In addition, FVH score was positively correlated with APCVs score, rΔφ (phase value) and NIHSSadmission. It suggests that the occurrence of FVH is related to cerebral oxygen metabolism.
Introduction
It has been proven that the occur of fluid-attenuated inversion recovery vascular hyperintensity (FVH) in brain should be due to the slow leptomeningeal collateral [1]. Prominent FVH-DWI (diffusion-weighted imaging) mismatch was associated with favorable outcome in acute infarctions in unilateral internal carotid artery occlusion patients [2]. Previous studies have suggested that the asymmetrical prominent cortical veins (APCVs) may be related with hypoperfusion or collateral flow in cerebral ischemia [3-4], but no consensus has been reached. Whether the appearance of FVH sign is associated with APCVs and whether there is relationship between FVH sign and cerebral oxygen metabolism need further study.Methods
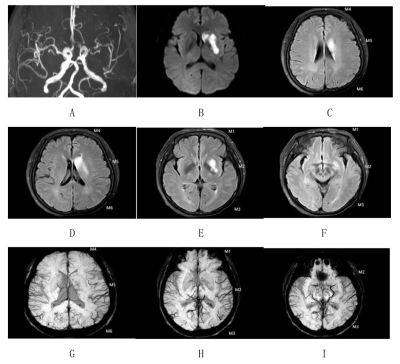
Twenty-eight acute or subacute unilateral MCA infarction patients were prospectively collected. The clinical base data of all patients were recorded, and the National Institute of Health stroke scale (NIHSS) scores at admission and discharge were evaluated (NIHSSadmission, NIHSSdischarged, Table 1). All MR images were acquired on a 3.0-T MRI scanner (Ingenia CX, Philips Healthcare, Best, the Netherlands) with an 8-channel receive-only head coil. All subjects underwent conventional MRI, DWI, SWI sequences. The FVHs on FLAIR images and APCVs on SWI were graded according the modified Alberta Stroke Program Early CT score (ASPECTS) system. All patients were divided into the mild-to-moderate stenosis and severe-occlusion groups. The phase values of venous were measured on the filtered phase images by SWI with signal processed on neuroimaging (SPIN) software.With reference to DWI images, the phase difference of cerebral internal vein was measured on SWI phase diagram, which was expressed by Δφ (phase) and ratio of Δφ between Δφlesion and Δφcontralateral (r∆φ) (Figure 1). All measurement were make independently by two radiologists. The differences of FVH and APCVs scores between the two groups were compared with independent t-test. Spearman correlation analysis were separately used to analyze the relationships of the FVH score with APCVs score, Δφ, rΔφ, NIHSS. The influencing factors of FVH score were analyzed by multiple linear regression.Results
In all patients, the FVH score (t = 3.13, p = 0.001) and APCVs score (t = 5.45, p = 0.001) in the severe-occlusion group were significantly higher than those in the mild-to-moderate group (Figure 2,Table 2). On unified comparison, the FVH score was positively correlated with APCVs score, r∆φ and NIHSSadmission (r = 0.60, p = 0.001; r = 0.39, p = 0.04; r = 0.43, p = 0.02, Figures 3-4). No significant correlation was observed between the FVH score and ∆φ, NIHSSdischarge (p > 0.05) whether overall or separately analysis. Multiple linear regression analysis showed that APCVs score was the main influencing factor of FVH score (b = 0.51, P = 0.01).Discussion
This study shows that the severe vascular stenosis were associated with increased FVH and APCVs scores, in a sense, this reflects the opening degree of collateral circulation and oxygen uptake. As for the mechanism of APCVs in ischemia, some studies demonstrated that the local ischemia could lead to venous dilatation, volume increase, and/or the uncoupling of oxygen supply and uptake [5-6]. Some studies indicated that APCVs is also related to collateral circulation. It is well known that the FVH sign can reflect collateral circulation in ischemic tissue, even if it has no consensus on prognosis. In this study, it was found that there was a correlation between the FVH and APCVs scores, indicating that both FVH sign and APCVs can used for description of collateral circulation state. The positive correlation between FVH score and r∆φ may suggest that, under the stimulation of ischemia and hypoxia, there was not only the opening of collateral circulation around the infarction core, but also the changes of cerebral oxygen metabolism, and these two mechanisms jointly compensated for tissue ischemia.Conclusion
The ranges of FVH and APCVs were observed to be related to the stenosis of the responsible vascular. The larger territory of FVH is consistent with more serious hypoxia and more serious damage of nerve function.Main Findings
Patients with severe-occlusion were associated with higher FVH and APCVs scores than those with mild-to-moderate stenosis. The FVH territory was observed to be positively correlated with APCVs territory, oxygen extraction in patients with cerebral infarction.Acknowledgements
No acknowledgement found.References
[1] Apfaltrer Paul, Wenz Holger, Böhme Johannes, et al. FLAIR Vascular Hyperintensities Indicate Slow Poststenotic Blood Flow in ICA Stenosis. [J] .Clin Neuroradiol, 2020, undefined: undefined.
[2] Yuan Tao, Ren Guoli, Hu Xianning, et al. Added assessment of middle cerebral artery and atrial fibrillation to FLAIR vascular hyperintensity-DWI mismatch would improve the outcome prediction of acute infarction in patients with acute internal carotid artery occlusion.[J] .Neurol Sci, 2019, 40: 2617-2624.
[3]Payabvash Seyedmehid, Taleb Shayandokht, Benson John C, et al. Susceptibility-diffusion mismatch in middle cerebral artery territory acute ischemic stroke: clinical and imaging implications.[J] .Acta Radiol, 2017, 58: 876-882.
[4]Yuan T , Ren G , Quan G , et al. Fewer peripheral asymmetrical cortical veins is a predictor of favorable outcome in MCA infarctions with SWI-DWI mismatch[J]. Journal of Magnetic Resonance Imaging, 2018.
[5]Haacke E M, Mittal S, Wu Z, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1.[J] .AJNR Am J Neuroradiol, 2009, 30: 19-30.
[6]Charlotte Rosso, Martin Belleville, Christine Pires,et al. Clinical usefulness of the visibility of the transcerebral veins at 3T on T2*-weighted sequence in acute stroke patients[J]. 81(6):0-1287.
Figures
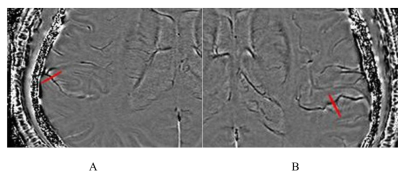
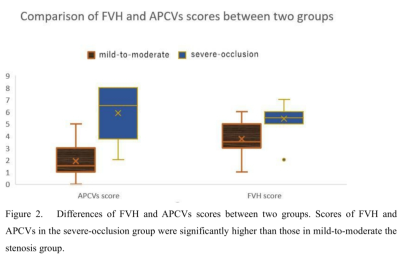
Figure 2. Differences of FVH and APCVs scores between two groups. Scores of FVH and APCVs in the severe-occlusion group were significantly higher than those in mild-to-moderate the stenosis group.