4320
Tractography analysis of dopamine transporter genetic mouse models in Parkinson's disease1Lee Gil Ya Cancer and Diabetes Institute, Incheon, Korea, Republic of
Synopsis
Parkinson's disease is characterized by degeneration of dopaminergic nigrostriatal neurons with dysfunctional cortico–striatal–thalamic loops mainly in the basal ganglia. However, Parkinson’s disease studies on the neural connections between brain structural regions have not reached a clear consensus on how Parkinson’s disease effects the mouse brain. In this study, probabilistic tractography analysis was performed on important mouse brain structures related to Parkinson's disease mechanism, and pathways between each domain were visualized.
Introduction
Parkinson Disease (PD) is a neurological disorder caused by abnormalities in the dopamine (DA) system, which in turn is known to affect both motor function and cognition1. As dopamine neurons in Substantia nigra (SN) degenerate in PD, the secretion of dopamine in the striatum (STR) decreases, resulting in a decrease in neuronal activity that triggers movement through the direct pathway, and the activity of neurons that inhibit movement through the indirect pathway2. Diffusion tensor imaging (DTI) tractography analysis can provide insight into the pathophysiology associated with dysfunction of major brainstem circuits, as it enables the evaluation of brainstem pathways3. In previous studies, studies on neural connectivity between areas of the Parkinson's brain structure have shown promising results, but are still quite incomplete4. In this study, we acquired DTI images of dopamine transporter (DAT) mice5, a Parkinson's disease model, using high-resolution 9.4T MRI, and conducted tractography analysis to investigate the connectivity between the structures of the direct and indirect pathways corresponding to the Parkinson's dopamine system.Material and Method
This study was conducted on a 9.4 T Bruker BioSpec horizontal bore, dedicated animal scanner (Bruker Biospin, Ettlingen, Germany), equipped with a gradient system of (660mT/m). For RF excitation a quadrature volume resonator (inner diameter (114mm); Bruker Biospin) was used. For signal reception, a quadrature mouse brain surface coil (Bruker Biospin) was applied. MRI data was acquired using Paravision 6.0 software. All experiments were performed on DAT mouse (3 months). Mouse transcardially were perfused and fixed with 4% paraformaldehyde and 0.1% Magnevist® in phosphate buffer (PB). Brains were extracted and incubated in 0.1% Magnevist/phosphate buffer for 4 days, placed in Fomblin and imaged. The pulse sequence used for this acquisition was 3D TurboRARE T2 (Spin echo sequence with a repetition time = 1800 ms, echo time = 37.7 ms, flip angle = 90°, Bandwidth = 99kHz, field of view = 1.2 × 1.2 × 1.6 cm, matrix = 200 × 200 × 265, resolution = 60 × 60 × 60 µm, 1 averages and resulting in a total acquisition time of 1h 44m) and 2D EPI-Diffusion tensor (Spin echo sequence with a repetition time = 3000 ms, echo time = 30 ms, flip angle = 90°, bandwidth = 171kHz, b-value = 3003 s/mm², diffusion gradient pulse duration (δ) = 4.5 ms, diffusion gradient separation (Δ) = 10.6 ms, diffusion direction = 30, field of view = 1.8 × 1.8 cm, slice thickness = 0.2 mm, matrix = 90 × 90, slice = 40, resolution = 200 x 200 x 200 µm, 8 averages and resulting in a total acquisition time of 2h 6m). Diffusion tensor image data were preprocessed by denoising and biasfield correction using MRtrix3. Region of interesting structures was processed using ANTx6-8 and FSL9. We acquired brain extracted images from whole-head input data and created masks based on Allen anatomical regions using ANTx. In addition, we acquired fiber reconstruction and probabilistic tractography data using FSL`s BEDPOSTX10 and PROBTRACKX11.Result
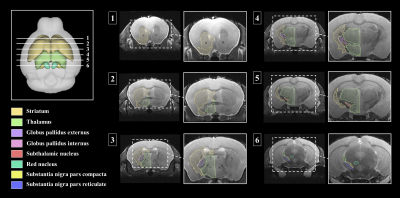
Brain segmentation analysis was performed in T2WI using ANTx, we visualized our segmentations on top of structural images to validate our results as shown in Figure 1. 3D visualization was done by partitioning the striatum(STR), thalamus(Thal), globus pallidus exterus(GPe), globus pallidus internus(GPi), subthalamic nucleus(STN), red nucleus(RN), substantia naigra compact part(SNc) and substantia naigra reticura(SNr) structures shown in Figure 2. The probabilistic tractography was used to compare the key areas connectivity between the control group and the PD group. The probabilistic tractography analysis is presented in the form of a linked matrix (Figure 3-A), with the results of Mann-Whitney U test comparing each group's connectivity strength (Figure 3-B). Connectivity map of key area structures was estimated between 32 anatomic regions with a log10 scale color map using waypoints connectivity. Figure 4 shows radar charts comparing probabilistic tractography connection intensity of control and PD mouse key area. The labels of structures shown statistically significant differences are colored emerald. In addition, connectivity between structures that show the significant difference from the Mann-Whitney U test statistical analysis are 3D rendered and visible in Figure 5.Discussion and conclusion
Key structures of parkinson disease were segmented in our study. The eight segmented areas (e.g., GPi, GPe, SNc, SNr, STN, RN, STR and Thal) were overlaid in T2WI to evaluate if the structures were in the correct position12. The tractography analysis results shows that the connectivity intensity between structures at a closer distance was stronger than between structures at farther distances13. In addition, out of the pathways showing statistically significant differences, the SNr connectivity was the most pronounced. Significant results also showed connectivity to the striatum in the direct path was decreased, and the connectivity to the thalamus in the indirect path was increased. The results of this study confirm that there is a difference in the strength of the network in key areas between the control group and the PD group, and it may reflect the degeneration of dopaminergic nigrostriatal neurons with dysfunction of the cortico–striatal–thalamic loops, a representative mechanism of Parkinson's disease14. These results will be useful in distinguishing image markers of motor function and cognitive degradation in Parkinson's disease and may serve as reference for further research with PD patients.Acknowledgements
This research was supported by Brain Research Program (NRF-2017M3C7A1044367) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT.References
[1] Savitt JM, Dawson VL, Dawson TM et al. Diagnosis and treatment of Parkinson disease: molecules to medicine. The Journal of clinical investigation. 2006;116(7):1744-1754.
[2] Suarez LM, Solis O, Sanz‐Magro A et al. Dopamine D1 Receptors Regulate Spines in Striatal Direct‐Pathway and Indirect‐Pathway Neurons. Movement Disorders. 2020;35(10):1810-1821.
[3] Zhang Y, Vakhtin AA, Jennings JS et al. Diffusion tensor tractography of brainstem fibers and its application in pain. PloS one. 2020;15(2):e0213952.
[4] Lan-xiang L, Dan D, Tao Z, et al. Detecting dopaminergic neuronal degeneration using diffusion tensor imaging in a rotenone-induced rat model of Parkinson's disease: fractional anisotropy and mean diffusivity values. Neural regeneration research. 2017;12(9):1485.
[5] Efimova EV, Gainetdinov RR, Budygin EA et al. Dopamine transporter mutant animals: a translational perspective. Journal of neurogenetics. 2016;30(1):5-15.
[6] Koch S, Mueller S, Foddis M, et al. Atlas registration for edema-corrected MRI lesion volume in mouse stroke models. Journal of Cerebral Blood Flow & Metabolism. 2019;39(2):313-323.
[7] Huebner NS, Mechling AE, Lee HL, et al. The connectomics of brain demyelination: functional and structural patterns in the cuprizone mouse model. Neuroimage. 2017;146:1-18.
[8] Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007; 445(7124):168.
[9] Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5(2):143-156.
[10] Jbabdi S, Sotiropoulos SN, Savio AM et al. Model‐based analysis of multishell diffusion MR data for tractography: How to get over fitting problems. Magnetic resonance in medicine. 2012;68(6):1846-1855.
[11] Behrens TEJ, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2003;50(5):1077-1088.
[12] Oishi K, Mori S, Troncoso JC et al. Mapping tracts in the human subthalamic area by 11.7 T ex vivo diffusion tensor imaging. Brain Struct Funct. 2020;225(4):20.
[13] Cacciola A, Calamuneri A, Milardi D et al. A connectomic analysis of the human basal ganglia network. Frontiers in neuroanatomy. 2017;11:85. [14] Hacker CD, Perlmutter JS, Criswell SR et al. Resting state functional connectivity of the striatum in Parkinson’s disease. Brain. 2012;135(12):3699-3711.
Figures