4318
Microstructural Properties of Major White Matter Tracts in Constant Exotropia before and after Strabismus Surgery1Hefei National Lab for Physical Sciences at the Microscale and the Centers for Biomedical Engineering, University of Science and Technology of China, Hefei, China, 2Department of Ophthalmology, The First Affiliated Hospital of Anhui Medical University, Hefei, China, 3Department of Ophthalmology, The People's Hospital of Bozhou, Bozhou, China
Synopsis
This study apply AFQ, in conjunction with MRtrix3 and ConTrack, to identify 24 major white matter fiber bundles in HCs, patients with XT before and after strabismus surgery. Meanwhile, we evaluate ocular dominance (OD) and stereo acuity of post-operative patients. We found that microstructures (MD) of vision-related fibers changed after surgery. Moreover, these changes were associated with indicators of OD. We infer that microstructural changes of the visual spatially related fiber bundles might contribute to the restoration of stereopsis, and the balanced binocular input may be more conducive to the improvements and restoration of binocular visual function.
Introduction
Strabismus is characterized by one eye’s visual axis deviation relative to the other.1 While the white matter (WM) microstructural deficits have been explored in strabismic amblyopia,2 microstructural changes in pre- and post-surgery for patients with strabismus have not been investigated. Consequently, this study aims to systematically investigate the differences in WM tracts between patients with constant exotropia (XT, a specific subtype of strabismus) and healthy controls (HCs), and the alterations before and after surgery. On the other hand, ocular dominance (OD) was revealed to be abnormal in patients with strabismus in 3 4, which shows that the weak eye was suppressed by the good eye. OD still remains abnormal in surgically corrected strabismus with normal clinical stereopsis and visual acuity.4 5 We thus intend to explore whether the two eyes contribute equally to binocular perception after surgery for XT, and further investigate the association between microstructural alterations and the OD. Notably, patients recruited in the present study have no stereovision prior to surgery. Moreover, they all recovered successfully from stereopsis following the surgical therapy. Therefore, we attempt to explore potential neural mechanism of postoperative stereopsis recovery.Methods
Nineteen (9 males; mean ± SD age: 23.53 ± 5.16 years) patients with XT from the Ophthalmology Department of the First Affiliated Hospital of Anhui Medical University were recruited. 20 age and gender-matched HCs were also recruited (10 males; mean ± SD age: 23.75 ± 5.48 years). All 19 patients underwent strabismus surgery. However, 4 patients did not attend post-surgery MRI scanning. Out of the remaining 15 patients, 4 patients’ OD was not measured. MRI was performed at the Information Science Center of University of Science and Technology of China, using a GE 3.0T scanner (Discovery™ MR750) equipped with a standard 8-channel head coil. Pre- and post-surgery images were collected with a time interval of 5.59 (±1.86) months. Diffusion-weighted images were acquired for 64 different directions of diffusion weighting. The b-value was set to 1000 s/mm2. Five nondiffusion-weighted images (b = 0) were acquired at the beginning of each scan. Imaging protocols were as follows: TR = 6900 ms, TE = 60.4 ms, flip angle = 90°, matrix size = 128 × 128, slice thickness = 2 mm, number of slices = 69, and voxel size = 2 × 2 × 2 mm3. 20 fiber tracts were identified from streamlines generated by MRtrix3 and selected by LiFE using the automated fiber quantification (AFQ) software package (https://github.com/yeatmanlab/AFQ/wiki).6 In addition, we identified the optic radiation (OR) and vertical occipital fasciculus (VOF) using probabilistic fiber tractography based on ConTrack (https://github.com/vistalab/contrack),7 which calculates the most likely pathway between a pair of regions of interest (ROIs). Each tract was resampled to 100 equidistant nodes. For each subject, we calculated the mean value of tract properties (FA and MD) across the middle 80 nodes in each tract. The two-sample t-test was used to evaluate differences between the preoperative and HC groups in these diffusion properties. The paired-sample t-test was employed to examine the possible changes in these properties before and after surgery. All analyses were corrected for multiple comparisons using false discovery rate (FDR) at p < 0.05. Furthermore, we also evaluated the relationship between OD and the absolute value of altered microstructural parameters.Results
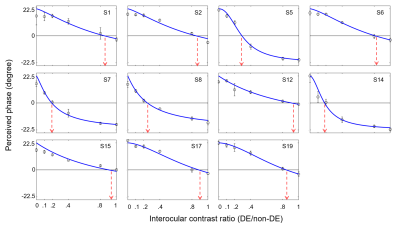
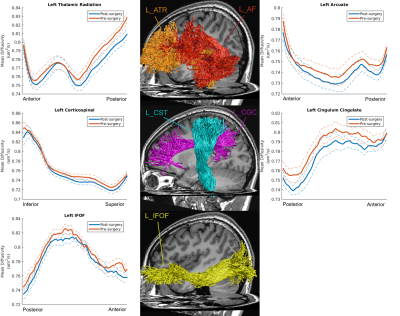
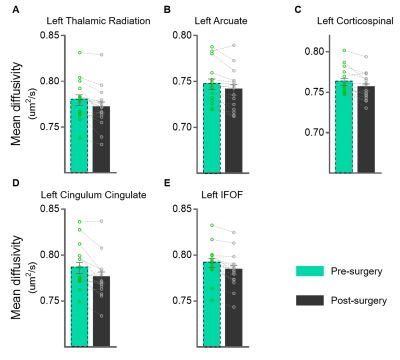
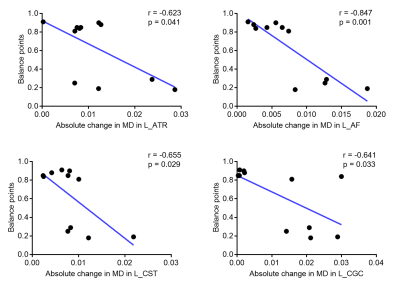
Our findings indicated that OD was still abnormal (i.e. balance point < 0.9) in most of post-surgical XTs (9 out 11), see Figure 1. There were no significant differences in FA or MD along all tracts in pre-surgery and HCs (FDR-corrected). The MD of post-surgery was significantly smaller than that of pre-surgery along left anterior thalamic radiation (ATR), left arcuate fasciculus (AF), left corticospinal tract (CST), left cingulum cingulate (CGC), and left inferior fronto-occipital fasciculus (IFOF), see Figures 2 and 3. Moreover, the balance points negatively correlated with the absolute value of MD alterations along left ATR (r=-0.623, p=0.041), left AF (r=-0.847, p=0.001), left CST (r=-0.655, p=0.029), and left CGC (r=-0.641, p=0.033) (uncorrected), see Figure 4. These correlation results suggest a larger balance point links to a smaller MD change in the fiber bundles.Discussion and Conclusion
Some studies have suggested that AF and CGC are involved in visuospatial processing,8-10 which may be responsible for postoperative stereopsis recovery. The IFOF possibly play an important role in visual processing, attention, and reading.11 Thus, the MD changes of left IFOF may be associated with improved visual reception and reading function of postoperative XT. Earlier research has linked the excitability of CST with visuomotor tasks.12 ATR is functionally connected to the dorsomedial thalamic nucleus which plays a key role in oculomotor control.13 Therefore, the improvement of eye movement function after strabismus surgery may be linked with microstructural changes of left CST and left ATR. Additionally, a negative correlation observed in our results between balance point, a measure of a balanced input, and changes in MD for vision-related fibers could suggest that larger microstructural changes might be required to restore, maintain, or improve the stereopsis and other visual functions as a brain compensatory mechanism following a loss of a balanced input. We, therefore, hypothesize that better binocular outcomes may be obtained when the OD is corrected after surgical correction.Acknowledgements
The authors are grateful to all study participants.References
1. Bui Quoc E, Milleret C. Origins of strabismus and loss of binocular vision. Front Integr Neurosci 2014;8:71. doi: 10.3389/fnint.2014.00071 [published Online First: 2014/10/14]
2. Duan Y, Norcia AM, Yeatman JD, et al. The Structural Properties of Major White Matter Tracts in Strabismic Amblyopia. Invest Ophthalmol Vis Sci 2015;56(9):5152-60. doi: 10.1167/iovs.15-17097 [published Online First: 2015/08/05]
3. Kwon M, Lu ZL, Miller A, et al. Assessing Binocular Interaction in Amblyopia and Its Clinical Feasibility. PLoS One 2014;9(6) doi: ARTN e100156 10.1371/journal.pone.0100156
4. Zhou J, Wang Y, Feng L, et al. Straightening the Eyes Doesn't Rebalance the Brain. Front Hum Neurosci 2017;11:453. doi: 10.3389/fnhum.2017.00453 [published Online First: 2017/09/29]
5. Feng L, Zhou J, Chen L, et al. Sensory eye balance in surgically corrected intermittent exotropes with normal stereopsis. Sci Rep 2015;5:13075. doi: 10.1038/srep13075 [published Online First: 2015/08/20]
6. Yeatman JD, Dougherty RF, Myall NJ, et al. Tract Profiles of White Matter Properties: Automating Fiber-Tract Quantification. PLoS One 2012;7(11) doi: ARTN e49790 10.1371/journal.pone.0049790
7. Sherbondy AJ, Dougherty RF, Ben-Shachar M, et al. ConTrack: Finding the most likely pathways between brain regions using diffusion tractography. Journal of Vision 2008;8(9) doi: Artn 15 10.1167/8.9.15
8. Carter AR, McAmoy MP, Siegel JS, et al. Differential white matter involvement associated with distinct visuospatial deficits after right hemisphere stroke. Cortex 2017;88:81-97. doi: 10.1016/j.cortex.2016.12.009
9. Thiebaut de Schotten M, Tomaiuolo F, Aiello M, et al. Damage to White Matter Pathways in Subacute and Chronic Spatial Neglect: A Group Study and 2 Single-Case Studies with Complete Virtual In Vivo Tractography Dissection. Cereb Cortex 2014;24(3):691-706. doi: 10.1093/cercor/bhs351
10. Mahayana IT, Tcheang L, Chen CY, et al. The precuneus and visuospatial attention in near and far space: a transcranial magnetic stimulation study. Brain Stimul 2014;7(5):673-9. doi: 10.1016/j.brs.2014.06.012 [published Online First: 2014/08/13]
11. Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008;44(8):1105-32. doi: 10.1016/j.cortex.2008.05.004
12. Morin-Moncet O, Therrien-Blanchet JM, Ferland MC, et al. Action Video Game Playing Is Reflected In Enhanced Visuomotor Performance and Increased Corticospinal Excitability. PLoS One 2016;11(12) doi: ARTN e0169013 10.1371/journal.pone.0169013
13. Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. Journal of Neurophysiology 2004;91(3):1381-402. doi: 10.1152/jn.00738.2003
Figures