4315
Cognitive and motor topography of human dentate nuclei identified with tractography and clustering methods1Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy, 2Brain Connectivity Center Research Department, IRCCS Mondino Foundation, Pavia, Italy, 3Department of Physics, University of Pavia, Pavia, Italy, 4Department of Electrical, Computer and Biomedical Engineering, University of Pavia, Pavia, Italy, 5Advanced Imaging and Radiomics Center, Neuroradiology Department, IRCCS Mondino Foundation, Pavia, Italy, 6NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London (UCL), London, United Kingdom
Synopsis
The dentate nuclei (DNs) represent the main output relay of the cerebellum and yet are often unexplored especially in human studies. For the first time, we used both tractography and an unsupervised fuzzy c-means classification algorithm to identify DNs topography on the basis of their connectivity to cerebellar and cerebral areas as well as their microstructural features. Our findings indicate that DNs can be parcellated in two main areas: one predominant non-motor representation and one motor representation. Furthermore, connectivity-based and microstructure-based atlases provide complementary information. These results represent a step-forward that could help interpretating pathological conditions involving cerebro-cerebellar circuits.
Introduction
Deep gray matter nuclei represent the synaptic relays of the brain and are responsible to route signals between specific brain areas. Cerebellar nuclei are crucial hubs for cerebro-cerebellar and spino-cerebellar communication. In particular, the dentate nuclei (DNs) represent the main output channel of the efferent cerebellar-thalamic connections and yet are often unexplored especially in human studies. The DNs are known to be involved in sensorimotor processes but recent functional imaging investigations have revealed that they play a role also in non-motor functions1–3. This finding has been also confirmed with tract-tracing4–6 and diffusion MRI tractography studies7–9, which showed that the cerebellum is connected to cognitive and associative cortical areas.Here, we developed a whole-brain multimodal MRI approach to identify DNs topography on the basis of their connectivity to cerebellar areas and subthalamic nuclei as well as their microstructural features.Materials and Methods
SubjectsTwenty-five subjects (16/9 female/male; 26-35 years) acquired using a Siemens 3T Connectome Skyra scanner were included. Pre-processed 3DT1-weighted images (0.7mm isotropic resolution resampled at 1.25mm) and high-quality diffusion-weighted data (1.25mm isotropic resolution, b=1000,2000,3000s/mm2, 90 isotropically distributed directions/shell and 18 b0 images) were downloaded from the ConnectomeDB (http://db.humanconnectome.org)10.
Preprocessing and whole-brain tractography
Diffusion tensor (DT) derived metrics, such as fractional anisotropy (FA), mean, axial and radial diffusivity (MD, AD, RD), were calculated with FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). Mean, axial and radial kurtosis (MK, AK, RK) maps were extracted with DESIGNER (https://github.com/NYU-DiffusionMRI/DESIGNER).
3DT1-weighted images were wrapped to the MNI152 template and segmented (FSL, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki) as white matter, gray matter (GM), subcortical GM, and cerebrospinal fluid. DN masks, previously extracted from diffusion data using a convolutional neural network (CNN)11, were added to the deep GM mask to improve the reliability of the segmentation and tractography. WM-GM interface was calculated for dynamically seeding a probabilistic whole-brain Anatomically-Constrained Tractography12–14 (MRtrix3, http://www.mrtrix.org/) (30 millions of streamlines).
Connectivity-based topography of the DN
For each subject, the SUIT atlas15 and the thalamus atlas16,17 were warped to each subject space. Six parcellations were defined both for the cerebellar hemispheres and thalami and numerical labels assigned to each one of them. Furthermore, a NOT-ROI was placed in the medial plane of the cerebellum. From the whole-brain tractography, cerebellar lobule-specific tracts were selected using the identified cerebellar parcellation and the ipsilateral DN, while subthalamic-specific tracts were selected using the DN and the contralateral subthalamic areas as target ROIs.For each subject, the cerebellar lobule-specific and subthalamic-specific topography atlases of DNs were created associating to each DN voxel numerical label of the cerebellar/thalamic parcellation that was connected to it with the highest number of streamlines.
Microstructure-based topography of the DN
For each subject, all diffusion-derived metrics were masked with the DN masks to obtain a dataset of microstructural features: each data point represented a voxel of the DN, with a vector of features per voxel generated from maps of different microstructural proprieties. Each feature of the dataset was normalized with respect to the group mean and outliers were replaced. This resulting dataset was inputted in an unsupervised fuzzy c-means clustering algorithm (MATLAB, https://www.mathworks.com/) aiming at identifying 2 main parcellations (=numerical label).
Inter-subject atlases of the DNs and comparison
DN topography maps of all subjects were warped to a common space (www.diedrichsenlab.org/imaging/suit_function.htm#norm_dentate) and inter-subject DN topography atlases were created assigning, to each voxel of the DNs, the mode of the numerical label across all subjects. To investigate the reproducibility between the different DN topography atlases (standard space), the DICE similarity coefficient (DSC) was computed.
Statistical analysis
For each subject and DN atlas, volumes of all parcellations were calculated. After tested for normality (Shapiro-Wilk test), a non-parametric Wilcoxon paired test was applied to assess statistically significant differences between the left and right side (SPSS v21, https://www.ibm.com/it-it/analytics/spss-statistics-software).
Results
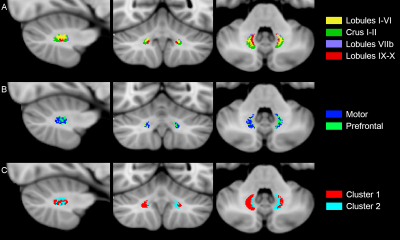
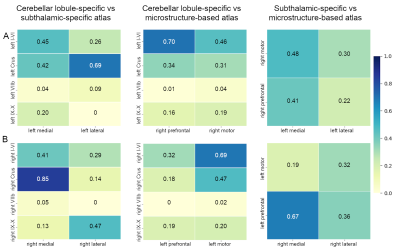
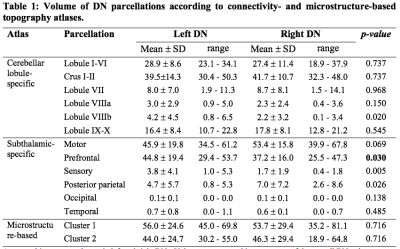
The inter-subject DN atlas based on cerebellar lobule-specific connectivity identified three main parcellations (Figure1A): one cognitive, one motor, and one sensory parcellation (respectively 40%, 30%, 15% of the DN volume). The DN atlas based on subthalamic-specific connectivity identified only two parcellations (Figure1B): one motor and one non-motor, indirectly connected with the prefrontal cortex (respectively 46%, 40% of the DN volume). Finally, the microstructure-based DN atlas distinguished one lateral and one medial parcellations (respectively 55%, 45% of the DN volume) (Figure1C). The three DN topography atlases showed no significant laterality effects (Table1). Regarding the comparison between different DN atlases (Figure2), the highest DSC value (0.85) was found between the right cognitive parcellation from the cerebellar-based atlas and the lateral parcellation from the microstructure-based atlas. Higher DSC are represented with more blue values (see color scale in Figure2).Discussion and Conclusions
This is the first study that identified the structural organization of the human DNs on the basis of their own connectivity both with the cerebellar and cerebral cortex (indirectly through the thalami) and exploiting their microstructural features. Our findings suggest that DNs can be consistently parcellated in two main distinct regions: one predominant non-motor representation and one motor parcellation. Although connectivity-based and microstructural-based parcellations were partially in agreement, it is to note that the resulting atlases contained different and complementary information. These results represent a step-forward for the interpretation of pathological conditions involving cerebro-cerebellar circuits.Acknowledgements
Data were provided by the Human Connectome Project, WU-Minn Consortium (PI: David Van Essen and Kamil Ugurbil; 1U54MH091657). ED and FP receive funding from the H2020 Research and Innovation Action Grants Human Brain Project 785907 and 945539 (SGA2 and SGA3), ED receives funding from the MNL Project “Local Neuronal Microcircuits” of the Centro Fermi (Rome, Italy), CGWK receives funding from the UK MS Society (#77), Wings for Life (#169111), Horizon2020 (CDS-QUAMRI, #634541), BRC (#BRC704/CAP/CGW).References
1. Alahmadi AAS, Pardini M, Samson RS, et al. Cerebellar lobules and dentate nuclei mirror cortical force-related-BOLD responses: Beyond all (linear) expectations. Hum. Brain Mapp. 2017;38(5):2566–2579.
2. Bharti K, Khan M, Beaulieu C, et al. Involvement of the dentate nucleus in the pathophysiology of amyotrophic lateral sclerosis: A multi-center and multi-modal neuroimaging study [Internet]. Neuroimage Clin 2020;28(April):102385.Available from: https://doi.org/10.1016/j.nicl.2020.102385
3. Zhang HY, Tang H, Chen WX, et al. Mapping the functional connectivity of the substantia nigra, red nucleus and dentate nucleus: A network analysis hypothesis associated with the extrapyramidal system. Neurosci. Lett. 2015;606:36–41.
4. Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. [Internet]. Brain 2006;129(Pt 2):290–2.[cited 2013 Feb 27 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16434422
5. Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. [Internet]. J Neurosci 2003;23(23):8432–44.Available from: http://www.ncbi.nlm.nih.gov/pubmed/12968006
6. Strick PL, Dum RP, Fiez J a. Cerebellum and nonmotor function. [Internet]. Annu Rev Neurosci 2009;32:413–34.[cited 2012 Nov 2 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19555291
7. Palesi F, Tournier J-D, Calamante F, et al. Contralateral cerebello-thalamo-cortical pathways with prominent involvement of associative areas in humans in vivo. [Internet]. Brain Struct Funct 2015;220(6):3369–3384.[cited 2014 Aug 20 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25134682
8. Palesi F, De Rinaldis A, Castellazzi G, et al. Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas [Internet]. Sci Rep 2017;7(1):12841.Available from: https://doi.org/10.1038%2Fs41598-017-13079-8
9. Kim Y, Im S, Kim SH, Park GY. Laterality of cerebellar afferent and efferent pathways in a healthy right-handed population: A diffusion tensor imaging study. J Neurosci Res 2019;97(5):582–596.
10. Van Essen DC, Smith SM, Barch DM, et al. The WU-Minn Human Connectome Project: An overview [Internet]. Neuroimage 2013;80:62–79.Available from: http://dx.doi.org/10.1016/j.neuroimage.2013.05.041
11. Gaviraghi M, Savini G, Castellazzi G, et al. Automatic segmentation of dentate nuclei for microstructure assessment : example of application to temporal lobe epilepsy patients. bioxRiv 2020;
12. Smith RE, Tournier J-D, Calamante F, Connelly A. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. [Internet]. Neuroimage 2012;62(3):1924–38.[cited 2014 Mar 24 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22705374
13. Jeurissen B, Tournier JD, Dhollander T, et al. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data [Internet]. Neuroimage 2014;103:411–426.Available from: http://dx.doi.org/10.1016/j.neuroimage.2014.07.061
14. Tournier J-D, Calamante F, Connelly A. Improved probabilistic streamlines tractography by 2 nd order integration over fibre orientation distributions. In: Proc Int Soc Magn Reson Med. 2010 p. 1670.
15. Diedrichsen J, Balsters JH, Flavell J, et al. A probabilistic MR atlas of the human cerebellum. [Internet]. Neuroimage 2009;46(1):39–46.[cited 2011 Jun 25 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19457380
16. Behrens TEJ, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. [Internet]. Nat Neurosci 2003;6(7):750–7.Available from: http://www.ncbi.nlm.nih.gov/pubmed/12808459
17. Behrens TEJ, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. [Internet]. Magn Reson Med 2003;50(5):1077–88.[cited 2011 Jul 12 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/14587019
Figures