4307
DTI fiber tracking reveals positive effects of motor training on stroke recovery1Radiology, University Hospital Münster, Münster, Germany, 2Medical Faculty, Experimental Magnetic Resonance, Westfälische-Wilhelms-Universität Münster, Münster, Germany, 3Department of Neurology with Institute for Translational Neurology, University Hospital Münster, Münster, Germany
Synopsis
Ex vivo diffusion tensor imaging (DTI) was used to study the effects of motor training on interhemispheric connectivity after ischemic stroke in mice. Training increased the number of fibers of the corpus callosum by one third. No overall effect of lesion size on DTI parameters and general interhemispheric connectivity was observed. Trained animals with large lesions, however, had higher fiber counts and axial diffusivity compared to non-trained animals with similarly large lesions. A larger benefit of motor training on animals with more severe stroke is implied.
Background
Diffusion tensor imaging (DTI) samples voxel-based water diffusion anisotropy. In the brain, diffusivity and fractional anisotropy (FA) allow for the quantification of axonal integrity and myelination. DTI fiber tracking has been successfully used to non-invasively monitor brain plasticity accompanying motor training and post-stroke remodeling1,2. It holds a key advantage over other neurological tools, such as viral tracers, in that it is non-destructive and delivers exceptional quantitative information especially in ex vivo applications3.Interhemispheric connectivity is stimulated by motor activity and both, high degrees of interhemispheric connectivity and motor therapy have been positively associated with stroke recovery2,4. Here, we used ex vivo DTI fiber tracking to study the effects of post-stroke motor training on interhemispheric connectivity in a murine model.
Methods
Experiments were performed with C57BL/6J mice. Photothrombotic stroke was induced under anesthesia by injection with Rose Bengal (0.2 mL i.v.) and immediate 560 nm laser illumination of the exposed skull 0.5 mm right from the bregma for 20 min. Motorized running-wheel-training was initiated 48 h after ischemia with a 1-week habituation of gradually increasing wheel speed and exercise duration. Subsequently, the running-wheel-training was continued five times a week for 75 minutes per session over a total period of seven weeks (n = 7). A control group received no motor therapy (n = 7). Mice were transcardially perfused with ice-cold phosphate buffered saline (0.1 M, pH 7.0) followed by 4% paraformaldehyde (PFA). Brains were removed from the skull and kept in 4% PFA for four days at room temperature. Next, they were washed and incubated for another 24 h in an aqueous solution of contrast agent (2 mM Magnevist). Finally, brains were embedded in 1% low-melting agar, enriched with 2 mM Magnevist.Diffusion-weighted images of fixed brains were recorded in axial direction with a 9.4 T Bruker BioSpec MR scanner and a Bruker cryogenic surface coil. We used a 2D multi-slice, multi-shot EPI sequence with TE = 0.72 ms; TR = 11250 ms and an in-plane resolution of 0.07 mm. Images without diffusion gradients (b0 images) were followed by diffusion weighted images in 30 isotropic gradient directions (b = 1000 s/mm²; diffusion time = 10 ms; diffusion encoding duration = 4 ms). Lesion sizes were measured by manually outlining the ipsilateral cortical signal void in the FA map.
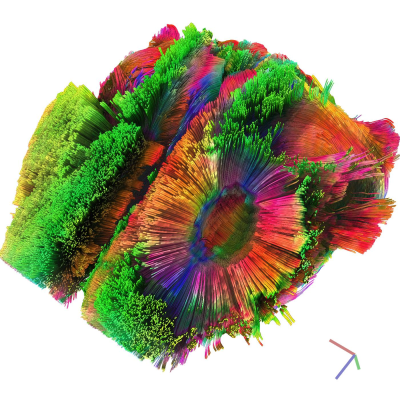
Diffusion tensor calculation and deterministic fiber tracking were performed with DSI-Studio5. Whole-brain seeding of 10000 sub-voxel seeds generated a tractographic overview of ~73000 tracts across individuals (FA tracking threshold = 0.08–0.10; angular threshold 60°; fiber length = 3–12 mm; RK4 algorithm with trilinear interpolation; exemplified in fig. 1). Seed regions of interest (ROI) in the corpus callosum were determined as detailed in fig. 2.
The effects of motor training on fiber count and on the diffusivity indices of the tracked fibers were assessed and statistical significance was tested with an unpaired t-test. Pearson correlation coefficients were determined between lesion dimensions and fiber parameters. The significance level alpha for all tests was 0.05.
Results and Discussion
Ischemic lesions were coherently found in the left primary somatosensory area. Lesion volumes were not significantly different between trained and non-trained animals.Short, radial fibers were reconstructed at the lesion contour (fig. 1). These structures are characteristic for post-stroke brains and are likely associated with perilesional astrocyte intrusion (gliosis, scar tissue) extending into the left primary and secondary motor areas2. On average, we found 36% more fibers in the reconstructed tracks of ROI A (exemplified in fig. 2C) of trained animals compared to non-trained animals. No difference in fiber counts was found in tracks passing ROI B and A (fig. 2D; fig. 3).
Animals with larger lesions usually had fewer interhemispheric tracks. Without training, significantly less fiber counts were found in animals with larger lesions. In contrast, after motor training, fiber counts were independent from lesion size (fig. 4A,B). Furthermore, large lesions were associated with higher axial diffusivity in fibers passing ROI B and A only in trained animals (fig. 4C). High axial diffusivity has previously been associated with improved post-stroke recovery6.
Tracking of the ipsilateral fibers adjacent to the ependymal zone of the anterior lateral ventricle was not possible when lesions penetrated into the left lateral ventricle. The accompanying damage to the ipsilateral ependyma is supposed to impair neuronal stem cell regeneration with negative impacts on post-stroke recovery7.
Our results highlight the utility of DTI fiber tracking for quantifying interhemispheric brain plasticity following motor training in a mouse stroke model. Furthermore, motor training appears to have the largest benefit for individuals with large lesions, i.e. more severe strokes.
Acknowledgements
No acknowledgement found.References
1) Islam M R, Luo R, Valaris S, Haley E B et al.: Diffusion tensor-MRI detects exercise-induced neuroplasticity in the hippocampal microstructure in mice. Brain Plasticity 2020;5:147-159.
2) Aswendt M, Pallast N, Wieters F, Baues M, Hoehn M, Fink G R: Lesion Size- and Location-Dependent Recruitment of Contralesional Thalamus and Motor Cortex Facilitates Recovery after Stroke in Mice. Translational Stroke Research 2020.
3) Calamante F, Tournier J-D, Kurniawan N D, Yang Z, Gyengesi E, Galloway G J, Reutens D C, Connely A: Super-resolution track-density imaging studies of mouse brain: Comparison to histology. NeuroImage 2012;59(1):286-296.
4) Heiss W-D: Contribution of Neuro-Imaging for Prediction of Functional Recovery after Ischemic Stroke. Cerebrovasc Dis 2017;44:266-276.
5) Yeh FC, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng WYI: Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLOS ONE 2013;8(11): e80713.
6) Moultin E, Mago S, Valabregue R, et al.: Acute Diffusivity Biomarkers for Prediction of Motor and Language Outcome in Mild-to-Severe Stroke Patients. Stroke 2019; 50(8).
7) Matta R, Gonzalez A L: Stroke Repair via Biomimicry of the Subventricular Zone. Front. Mater. 2018;5(15).
Figures