4304
Automatic tractography for brain tumor surgery: towards clinical application1TU/e Eindhoven University of Technology, Eindhoven, Netherlands, 2ETZ Elisabeth-TweeSteden hospital, Tilburg, Netherlands
Synopsis
We have introduced an automatic pipeline based on diffusion-weighted tractography for the reconstruction of four white-matter structures of interest. These anatomical reconstructions are visualized during brain tumour surgery to aid in the prevention of sensorimotor, visual and language deficits. The automatic tractography pipeline produces robust results, owing in part to the deep-learning based segmentation of brain regions which is robust to deformations of the brain due to brain shift, and the post-tractography filtering method which can remove spurious fibers.
Introduction
In previous work1 we have introduced an automatic pipeline based on diffusion-weighted tractography for the reconstruction of four white-matter structures of interest2. These anatomical reconstructions are visualized during brain tumour surgery to aid in the prevention of sensorimotor, visual and language deficits. The current work outlines the steps taken to bring the automatic pipeline to the clinical practice.Methods
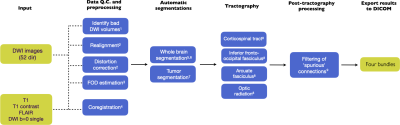
In Figure 1 an overview is given of the automatic tractography pipeline. Significant updates have been made by 1) further refining the tractography results by region-of-interest improvements based on feedback from clinicalexperts, 2) post-tractography filtering of spurious fibers using Fiber-To-Bundle Coherence (FBC) measures3, 3) incorporating automatic tumor segmentation, 4) developing a (REST-API based) user interface for controland visualization of the pipeline results, and 5) integrating with the PACS system by providing a DICOM output of the tractography results.Results
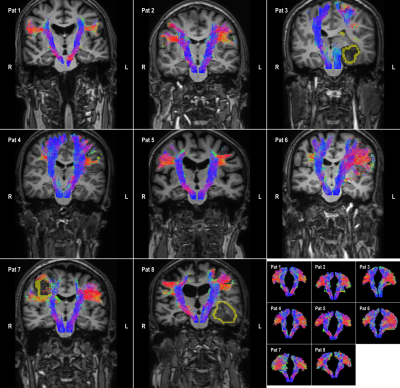
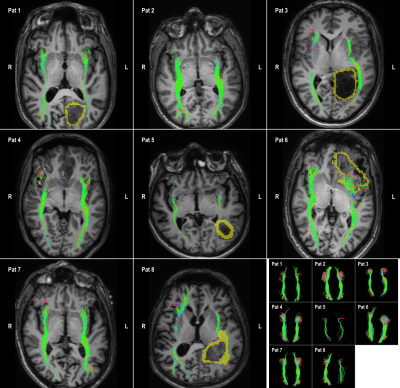
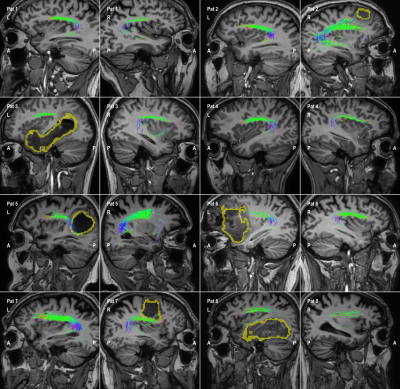
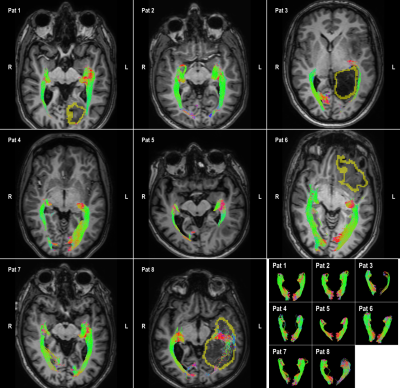
8 patients are included who were candidates for brain tumor surgery (low and high-grade gliomas). The data was acquired using a clinically-applicable diffusion weighted imaging protocol (b=1500, n=53) acquired on a 3T Philips Achieva Scanner. Figures 2 to 5 illustrate the results of automatic tractography for the corticospinaltract (CST), inferior fronto-occipital fasciculus (IFOF), arcuate fasciculus (AF), and the optic radiation (OR), respectively. The fibers are colored according to their orientation (red=L-R, green=A-P, blue=S-I) and the glioma is outlined in yellow. The majority of tracts were found to be consistent with anatomical knowledge as judged by clinical experts. The tractography results were disrupted in some cases, i.e. the IFOF for Pat 6 and 8 and the OR for Pat 3, 8 and 8.Discussion and conclusion
The automatic tractography pipeline produces robust results, owing in part to the deep-learning based SLANT segmentation of brain regions which is robust to deformations of the brain due to brain shift, and the FBC method which can remove spurious fibers. A possible downside of the FBC method is that it is necessary to specify a threshold for filtering, which is currently set conservatively but should ideally be tailored to the specific patient and take into account the disruptive effects of mass-effects and infiltration caused by the presence of a tumor. In future work the pipeline will be further evaluated in a non-inferiority study in which the tractography results are compared to the current clinical standard.Acknowledgements
This work is part of the research programme “Diffusion MRI Tractography with Uncertainty Propagation for the Neurosurgical Workflow” with project number 16338, which is (partly) financed by the Netherlands Organisation for Scientific Research (NWO).References
1Stephan Meesters, Geert-Jan Rutten, Andrea Fuster, and Luc Florack. Automated tractography of four white matter fascicles in support of brain tumor surgery. In Proceedings of the Organization for Human Brain Mapping annual meeting, 2019.
2G.J.M. Rutten, G. Kristo, W. Pigmans, J. Peluso, and H.B. Verheul. The use of MR-tractography in daily neurosurgicalpractice [in Dutch]. Dutch Journal of Neurology and Neurosurgery, 115:204-211, 2014.
3Stephan Meesters, Pauly Ossenblok, Louis Wagner, Olaf Schijns, Paul Boon, Luc Florack, Anna Vilanova, and Remco Duits.Stability metrics for optic radiation tractography: Towards damage prediction after resective surgery. Journal of NeuroscienceMethods, 288:34-44, 2017.
4J-Donald Tournier, Fernando Calamante, and Alan Connelly. MRtrix: Diffusion tractography in crossing fiber regions. Inter-national Journal of Imaging Systems and Technology, 22(1):53-66, 2012.
5Yuankai Huo, Zhoubing Xu, Katherine Aboud, Prasanna Parvathaneni, Shunxing Bao, Camilo Bermudez, Susan M. Resnick,Laurie E. Cutting, and Bennett A. Landman. Spatially localized atlas network tiles enables 3D whole brain segmentation from limited data. In Medical Image Computing and Computer Assisted Intervention MICCAI 2018, pages 698-705. Springer International Publishing, 2018.
6K. Niemann, V.R. Mennicken, D. Jeanmonod, and A. Morel. The Morel stereotactic atlas of the human thalamus: Atlas-to-MR registration of internally consistent canonical model. NeuroImage, 12(6):601-616, 2000.
7Guotai Wang, Wenqi Li, Sebastien Ourselin, and Tom Vercauteren. Automatic brain tumor segmentation using cascaded anisotropic convolutional neural networks. CoRR, abs/1709.00382, 2017.
Figures