4298
Surface-based Shape Morphometry Analysis of Human White Matter Tracts
Yi-Chia Kung1, Chu-Chung Huang2, Chih-Chin Heather Hsu1,3, Shin Tai Chong1, Ching-Po Lin1, and Chun-Hung Yeh4,5
1Institute of Neuroscience, National Yang-Ming University, Taipei, Taiwan, 2Institute of Cognitive Neuroscience, School of Psychology and Cognitive Science, East China Normal University, Shanghai, China, 3Center for Geriatrics and Gerontology, Taipei Veterans General Hospita, Taipei, Taiwan, 4Institute for Radiological Research, Chang Gung University and Chang Gung Memorial Hospital, Taoyuan, Taiwan, 5Department of Child and Adolescent Psychiatry, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan
1Institute of Neuroscience, National Yang-Ming University, Taipei, Taiwan, 2Institute of Cognitive Neuroscience, School of Psychology and Cognitive Science, East China Normal University, Shanghai, China, 3Center for Geriatrics and Gerontology, Taipei Veterans General Hospita, Taipei, Taiwan, 4Institute for Radiological Research, Chang Gung University and Chang Gung Memorial Hospital, Taoyuan, Taiwan, 5Department of Child and Adolescent Psychiatry, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan
Synopsis
This study proposes a novel framework for quantifying the alteration in shape morphometry of white matter (WM) fiber tracts. The surface of each WM bundle is segmented from diffusion MRI data using TractSeg, followed by surface reconstruction, parameterization, and modelling. With the M-rep parameterization and vertex-wise model distance, the proposed method was able to differentiate the level of morphological lateralization between bilateral arcuate fasciculi and cortical spinal tracts.
Introduction
The morphology of brain white matter (WM) tracts evolves over time, particularly during early brain development. Furthermore, functional plasticity has been proposed to be associated with structural plasticity that involves changes in WM physical structures. Hence, there has been a growing interest in shape analysis of WM fibers1,2. Recent technical advances provide reliable segmentation of WM fiber tracts based on diffusion MRI data on an individual basis3. Inspired by those methods, this study proposes a novel framework for surface morphometry analysis of WM fiber tracts, to enable measuring the alterations of WM tracts quantitatively via various vertex-wise metrics. In this study, we further examined the proposed technique for differentiating the level of morphological lateralization between bilateral arcuate fasciculus (AF) and cortical spinal tract (CST).Methods
Deformation analysis frameworkThe proposed technique is developed using an in-house script that integrates the functionalities of other software packages. There are five major procedures:
- DWI processing – An in-house toolkit (OGIO) was used to perform the current state-of-the-art DWI preprocessing based on MRtrix34 and FSL5 software tools. These steps include DWI denoising, Gibb’s ringing removal, corrections for image distortion, subject movement, and signal drift. Fiber orientation distributions (FODs) were then computed using multi-shell multi-tissue constrained spherical deconvolution6.
- Segmentation of WM fiber bundles – This is achieved by running TractSeg3 on the FODs from the previous step.
- Surface reconstruction of WM fiber bundles – the shapes of WM fiber bundles were modeled using surface meshes, which were generated using SPHARM-PDM7.
- Shape analysis of WM fiber bundles – For WM fiber bundle, the parameterization and analysis of the surface mesh were performed using SlicerSALT7.
- Statistical analysis – Our current implementation includes two types of group statistical analysis: a) Paired t-test can be used to assess the distance between the medial axis (M-rep)8of the WM bundle and per vertex. b) Following surface alignment using SPHARM-PDM algorithm, the vertex-wise model distances between any two surfaces are analyzed using the multivariate functional shape data analysis (MFSDA)9 to evaluate the statistical significance.
The proposed technique was applied to investigate the potential shape differences of bilateral AF (i.e. shape lateralization of WM tract); bilateral CSTs were also analyzed for comparisons. Our study cohort includes 74 normal ageing participants (age = 66.18±6.24 y/o, F/M = 39/35). DWIs were acquired at 3T Siemens Tim Trio MRI scanner using the simultaneous multi-slice EPI sequence (2-mm isotropic voxel, TR/TE=3525/109.2ms, multiband factor=3, DW gradient directions=30/60 at b-value=1000/3000s/mm2 respectively).
To conduct the group analysis, the study cohort was subdivided into 4 groups based on gender and age (using a threshold of 65 y/o). FOD templates were generated for all groups, from which a cohort FOD template was further generated. For group comparisons, each of the generated WM shape models was aligned to the corresponding one from the shape model of cohort template. The SPHARM-PDM algorithm was used to reorient and align the shape between hemispheres to enable statistical analysis.
Results
Using M-repThe M-rep analysis provides radius and area metrics, both of which were measured at each segment along the medial axis of the surface model. Figs. 2c & 3c showed that there were significant differences in both metrics (P<10-4) at various segments between bilateral AF; the right AF was significantly larger in the anterior-middle part but smaller in the posterior segment than the left AF. By contrast, bilateral CSTs presented a symmetric morphology with insignificant differences across all segments (Figs. 2d & 3d; P>0.05).
Using vertex-wise model distance
The vertex-wise analysis measures the distance from model to model. The distance implies the tract distribution in white matter bundle. The longer distance suggests the border tract distribution and vice versa. Fig. 4 shows the outcomes of vertex-wise comparisons between bilateral AF and CSTs, with statistical significance set at family-wise error corrected P<0.05. Overall, AF presented highly lateralized as compared to CSTs, which were consistent with the finding from the M-reap analysis.
Discussion
Our data demonstrate that the proposed framework can be used to study the surface morphology of WM fiber bundles. Previous studies have shown that AF lateralization appears to be common in the brain especially in elder adults10; the proposed method is able to capture such laterality in WM tracts. It is worth noting that the vertex-wise analysis can evaluate localized morphological variations of WM tracts and provide more information regarding tract profile, such as orientation and distribution than the typical M-rep model. Left AF presented a broader distribution in the temporal pole from the lateral view, which replicates previous findings10. Right AF tends to distribute to a broader range from the medial view, while left AF shrinks to a narrow form. Moreover, with vertex-wise deformation analysis, we could detect the significant lateralization in CST, which was not presented in the conventional M-rep parameterization. The longer distance was found in right than left of the top CST, the region associated with hand and leg manipulation. It may relate to that all the participants are right-handed.Conclusion
We introduce a novel framework for quantifying surface morphology of WM tracts. Investigations of the evolutionary WM morphological changes during brain development, aging, training, or disease should benefit from the proposed method.Acknowledgements
This study was supported in part by grants from Ministry of Science and Technology, Taiwan (MOST 108-2321-B-010-010-MY2, MOST 109-2222-E-182-001-MY3).References
- Yeh, F.-C., Shape analysis of the human association pathways. NeuroImage, 2020. 223: p. 117329.
- Chandio, B.Q., et al., Bundle analytics, a computational framework for investigating the shapes and profiles of brain pathways across populations. Scientific Reports, 2020. 10(1).
- Wasserthal, J., P. Neher, and K.H. Maier-Hein, TractSeg - Fast and accurate white matter tract segmentation. NeuroImage, 2018. 183: p. 239-253.
- Tournier, J.-D., et al., MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 2019. 202: p. 116137.
- Smith, S.M., et al., Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 2004. 23: p. S208-S219.
- Tournier, J.D., F. Calamante, and A. Connelly, Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. NeuroImage, 2007. 35(4): p. 1459-1472.
- Vicory, J., et al., SlicerSALT: Shape AnaLysis Toolbox, in Shape in Medical Imaging. 2018, Springer International Publishing. p. 65-72.
- Paniagua, B., et al., Lateral ventricle morphology analysis via mean latitude axis. Proceedings of SPIE--the International Society for Optical Engineering, 2013. 8672.
- Li, Y., et al., Multiscale adaptive regression models for neuroimaging data. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 2011. 73(4): p. 559-578.
- Lebel, C. and C. Beaulieu, Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human brain mapping, 2009. 30(11): p. 3563-3573.
Figures
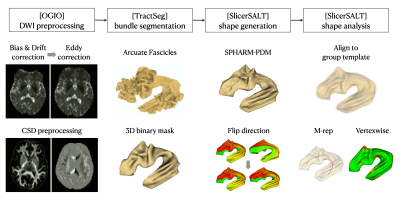
Figure 1. The procedure of surface-based shape morphometry analysis for the white matter tracts. It contains DWI pre-processing, white matter bundle segmentation, bundle shape generation, shape analysis with parameterization, and statistical analysis by M-rep and vertex-wise analysis.
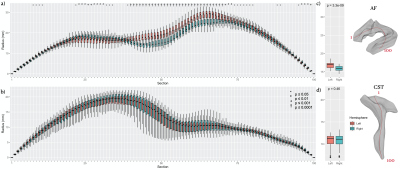
Figure 2. M-rep lateralization of radius in (a) AF and (b) CST for the segmented 1 to 100 portion, and the average radius in each tract (c, d). (red: tract in left hemisphere, blue: tract in right hemisphere, *: P-value ≤ 0.05. ª: P-value ≤ 0.01, +: P-value ≤ 0.001, †: P-value ≤ 0.0001)
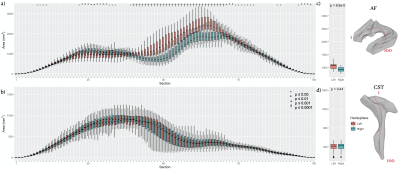
Figure 3. M-rep lateralization of area in (a) AF and (b) CST for the segmented 1 to 100 portion, and the average radius in each tract (c, d). (red: tract in left hemisphere, blue: tract in right hemisphere, *: P-value ≤ 0.05. ª: P-value ≤ 0.01, +: P-value ≤ 0.001, †: P-value ≤ 0.0001)
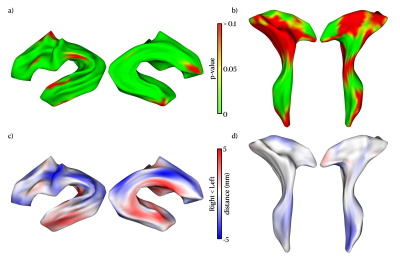
Figure 4. Vertex-wise analysis for the distance between the tract models of left and right hemispheres. The upper panel shows the distance significantly different from left to right shape model in (a) AF and (b) CST. (green: P-value ≤ 0.05, FWE corrected) The lower panel presents the vertex-wise differences between the shape model from left to right hemisphere in (c) AF and (d) CST. (red: left > right, blue: right > left).