4292
Enhancing tractography filtering by accounting for outliers and partial voluming in diffusion weighted measurements1Department of Computer Science, University of Verona, Verona, Italy, 2Translational Imaging in Neurology, Department of Medicine and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland
Synopsis
The white matter structures of the human brain can be represented via diffusion tractography. Unfortunately, tractography is prone to find false-positive streamlines causing a severe decline in its specificity and limiting its clinical feasibility. Filtering algorithms have been proposed to reduce these invalid streamlines. We augmented the COMMIT filtering algorithm to adjust for two typical artifacts present in diffusion-weighted images: partial voluming and signal drop-outs due to subject motion. We demonstrate that our robust algorithm is capable to properly filter tractography reconstructions despite these artifacts and could be useful especially for clinical studies with uncooperative patient groups such as neonates.
Introduction
Tractogram filtering is proposed to increase the specificity of tractography algorithms that generally produce numerous false positive streamlines1. The aim of this study was to improve one filtering algorithm, the Convex Optimization Modelling for Microstucture Informed Tractography (COMMIT)2,3, to cope with the presence of voxel-wise partial voluming effects as well as outliers4 in the original diffusion-weighted images (DWI). COMMIT uses the reconstructed streamlines and a microstructural model to predict DWIs. In this forward modeling, each streamline makes a contribution to the predicted DWI. Contributions are iteratively updated based on a cost function, e.g.,$$$L\left(\mathbf{y},\hat{\mathbf{y}}\right)=\mathbf{W}\parallel \mathbf{y}-\hat{\mathbf{y}} \parallel^2_2$$$, until the algorithm converges to a prediction $$$\hat{\mathbf{y}}$$$ that is the closest to the original measurements $$$\mathbf{y}$$$ by minimizing the root-mean-squared-error (RMSE) between them. Cost functions and RMSE can be weighted by a matrix $$$\mathbf{W}$$$ to account for data reliability.We augmented the cost function of COMMIT to adjust for voxel-wise reliabilities as weights. Such reliability could be for example a white-matter (WM) probability map5 or voxel-wise or slice-wise outlier probability map4. This approach could be helpful for any study using tractography, as partial voluming is an unavoidable issue with voxel size being larger than modelled brain structures. Moreover, we demonstrate that this approach is robust towards subject motion induced artifacts such as signal drop-out which is a common artefact in neonatal imaging.
Methods
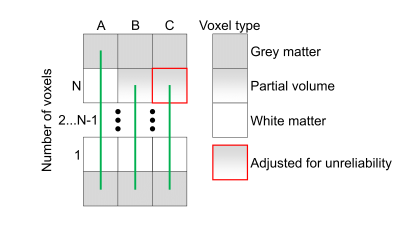
To study the effects of partial voluming, we built the simple synthetic phantom described in Fig. 1. The phantom consisted of: (A) a single streamline spanning through N WM voxels, (B) the Nth voxel was affected by partial voluming causing the streamline to end prematurely, and (C) the reliability of the last voxel was adjusted in the modelling to consider the partial voluming. Partial voluming was simulated as 70% of isotropic signal and the rest as WM. We repeated the phantom test with $$$N=[3...100]$$$.Ground-truth signal was simulated with the Stick-Ball model (WM/isotropic) implemented in dmipy6. The stick was simulated with parallel diffusivity $$$1.7\cdot10^{-9}\frac{\mathrm{m}^2}{s}$$$, and the isotropic diffusivity was $$$3.0\cdot10^{-9}\frac{\mathrm{m}^2}{s}$$$ with 3:1 signal fraction, i.e. ground-truth streamline should explain 0.75 of the signal. We used the Human Connectome Project’s (HCP) gradients to simulate the DWIs with 300 noise samples and b0 signal-to-noise ratio of 20.
In-vivo experiments to evaluate the robustness to partial voluming were conducted on one subject from HCP dataset (#103818). We generated a whole-brain tractogram with 3 million streamlines with MRtrix37 and filtered it with standard COMMIT and the proposed robust version using the Stick-Ball model. Voxel-wise reliabilities were estimated using SPM12 WM segmentation5 results.
Finally, we used a 3T MRI clinical neonatal dataset to investigate the effects of subject motion induced outliers in the tractogram filtering. The acquisition had two shells (b-values $$$60\times750\frac{\mathrm{s}}{\mathrm{mm}^2}$$$, $$$74\times1800\frac{\mathrm{s}}{\mathrm{mm}^2}$$$) and 13 $$$b=0\frac{\mathrm{s}}{\mathrm{mm}^2}$$$ . ExploreDTI8 with SOLID-plugin4 was used to process DWIs and to handle the slice-wise outliers throughout the pipeline. Whole-brain tractogram was calculated with MRtrix37 using 2 million streamlines and then filtered.
Results and Discussion
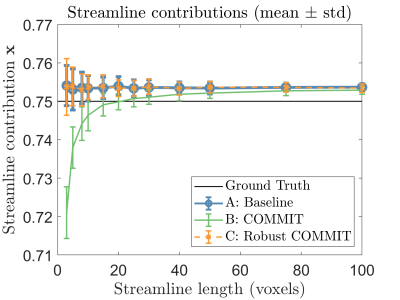
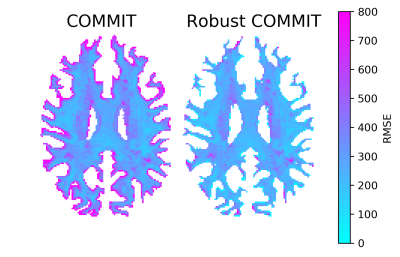
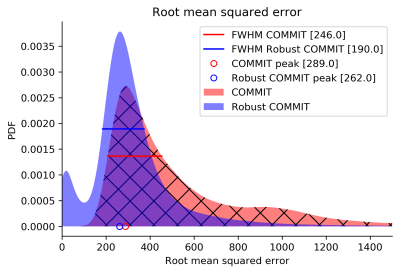
Fig. 2 shows the results of partial voluming tests with the synthetic phantom. In the baseline (A), the contribution of one streamline was nearly the theoretical (0.75) for all inspected tract lengths with a small offset of 1% due to noise. In (B) with the last voxel being artifactual, the streamline contribution was more severely affected in shorter streamlines. In (C) which was also affected by partial voluming but adjusted for unreliability, we obtained nearly identical results compared to the baseline and thus successfully handled this artifact.We evaluated the algorithm on one HCP subject by comparing the RMSE between the normal and robust filtering. In Fig. 3, an axial RMSE map is shown for both methods demonstrating that the normal filtering is producing large RMSE values at the GM/WM boundary where voxels are affected by partial voluming. By introducing WM probability map in robust filtering, this boundary area does not influence the filtering and potentially allows obtaining more realistic fiber contributions. Fig. 4 depicts the whole-brain histograms of the RMSE evaluation indicating that this correction improves the fitting throughout the whole WM.
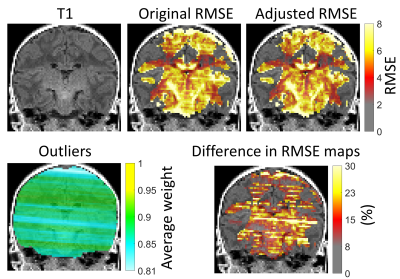
To prove that the proposed method could help to robustly analyze datasets containing motion induced artifacts, we performed preliminary tests with a neonatal dataset (Fig. 5). The original RMSE map is clearly affected by stripes matching the outlier positions whereas the adjusted robust RMSE is not. The effect is most prominent in the RMSE difference map (original > adjusted) that clearly shows how robust filtering decreases the RMSE and could provide more realistic estimates of the streamline contributions.
Conclusions
We presented a novel augmentation to the tractogram filtering algorithm COMMIT2,3 to adjust for outliers and artifacts that are often present in the DWIs acquired in clinical settings. Besides the unavoidable partial voluming, data acquired from uncooperative patients like neonates, elderly, acutely sick, is often corrupted also by outliers due to subject motion. Our robust augmentation of COMMIT considers both of these error sources. We evaluated the algorithm with simulations using synthetic phantom and HCP data with promising results as well as applied it to a clinical dataset as a proof of concept to demonstrate its clinical feasibility.Acknowledgements
This study was conducted using data from the Human Connectome Project (www.humanconnectome.org). VS was supported by the Brain Research Foundation Verona and the Emil Aaltonen Foundation.References
1. Maier-Hein, Klaus H., et al. "The challenge of mapping the human connectome based on diffusion tractography." Nature communications 8.1 (2017): 1-13.
2. Daducci, Alessandro, et al. "COMMIT: convex optimization modeling for microstructure informed tractography." IEEE transactions on medical imaging 34.1 (2015): 246-257.
3. Schiavi, Simona, et al. "A new method for accurate in vivo mapping of human brain connections using microstructural and anatomical information." Science advances 6.31 (2020): eaba8245.
4. Sairanen, Viljami, Alexander Leemans, and Chantal MW Tax. "Fast and accurate Slicewise OutLIer Detection (SOLID) with informed model estimation for diffusion MRI data." Neuroimage 181 (2018): 331-346.
5. Ashburner, John, and Karl J. Friston. "Unified segmentation." Neuroimage 26.3 (2005): 839-851.
6. Fick, Rutger HJ, Demian Wassermann, and Rachid Deriche. "The dmipy toolbox: Diffusion mri multi-compartment modeling and microstructure recovery made easy." Frontiers in neuroinformatics 13 (2019): 64.
7. Tournier, J-Donald, et al. "MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation." NeuroImage 202 (2019): 116137.
8. Leemans, A., Jeurissen, B., Sijbers, J. & Jones, D. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc. 17th Sci. Meet. Int. Soc. Magn. Reson. Med. 17, 3537 (2009).
Figures