4270
A wearable “RF-EEG Cap” for full head coverage concurrent TMS/EEG/fMRI experiments at 3T: a feasibility study1Martinos Center - MGH, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3High Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria
Synopsis
To enable concurrent non-invasive stimulation (TMS) and whole-head multimodal imaging (EEG-fMRI), we propose to apply flexible RF coil technology to build a first-of-its-kind TMS compatible integrated multimodal imaging array, the “RF-EEG cap”. The proposed system allows unrestricted positioning of the TMS coil across the entire scalp. We built a 2-channel prototype and conducted a feasibility study analyzing the effects of a TMS coil and EEG-electrodes on the imaging quality (SNR/B0 maps), the in-bore EEG data quality and EPI timeseries stability. Our results indicate that the flexible coaxial RF technology is a feasible choice to build the proposed “RF-EEG Cap”.
Introduction
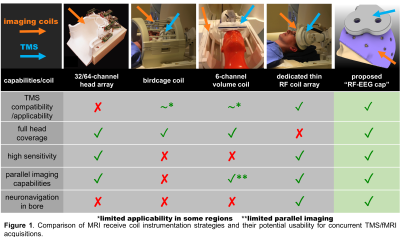
There is mounting evidence that the effects of TMS and its therapeutic efficacy depend on the primary stimulation target region as well as its connectivity pattern (spatial dependency)1,2. Additionally, the neuromodulation effects depend on the brain state at the time of stimulation (time dependency)3 defined by neural oscillations. Combining TMS/fMRI/EEG offers the next-generation capabilities for causal-functional mapping of the human brain circuits in a non-invasive way with unprecedented spatial and temporal resolution potentially enhancing therapeutic protocols through closed-loop applications. However, the triple combination presents technological challenges especially due to the lack of dedicated hardware to allow optimal performance of TMS and MRI (see Fig.1 column 1-4).Feasibility of concurrent human TMS/EEG/fMRI measurements at 3T has been recently demonstrated4,5. However, the presented data were acquired either with the body coil (extremely low-sensitivity) or a birdcage coil (Fig.1 column 2). Moreover, no parallel imaging acquisition methods6,7,8 were used due to the lack of multichannel RF-coils, limiting the spatiotemporal resolution of the acquisition. Nevertheless, these results rigorously demonstrate the basic feasibility and safety of the technology.
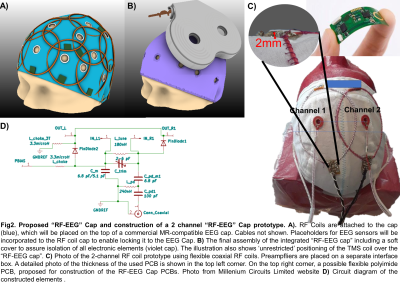
Our overarching goal is to build the first-of-its-kind wearable “RF-EEG Cap” to allow full-head coverage concurrent TMS/fMRI/EEG acquisition (Fig.1, column 5, Fig.2A and 2B). Here, to assess the feasibility of the proposed “RF-EEG Cap” we have constructed a 2-channel prototype shown in Fig.2C.
Methods
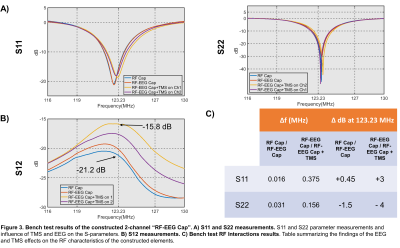
For the prototype construction we implemented flexible coaxial RF elements9,10 easily attachable to soft materials. We built two 8cm diameter coils using flexible coaxial cable (Molex 047SC-2901, IL, USA) following proposed tuning/matching circutry11 (see Fig.2D). Active detuning and preamplifier decoupling were implemented. Both elements were sewn onto a cloth cap and connected through 22.5 cm coaxial cables to an interface box containing one 3T preamplifier (Siemens, Germany) and a 3T Skyra plug. Placeholders for EEG-electrodes in the middle of each element were made on the cap to facilitate the positioning.The prototype was tested on the Bench using a VNA (Keysight Technologies, CA, USA) as: (i) stand-alone (only having RF elements), referred as RF-Cap; (ii) placing two bipolar MR compatible EEG-electrodes (Brain Products GmbH, Germany), referred as RF-EEG Cap and (iii) placing the EEG-electrodes and the MRi B91 TMS coil (MagVenture, Denmark) over the cap, referred as RF-EEG Cap+TMS.
To evaluate the effects on the imaging of the two additional modalities, we acquired SNR maps (FOV=256 mm, 1 slice, FA = 30, TR= 9.1 ms, TE=4.8 ms, SL= 2mm, 1 mm in-plane) and B0 maps (FOV=220mm, 18 slices, FA=75, TR=300ms, TE1=5ms, TE2=7.46ms, SL=5mm, 2.2mm in-plane) which were processed with in-house MATLAB scripts for visualization.
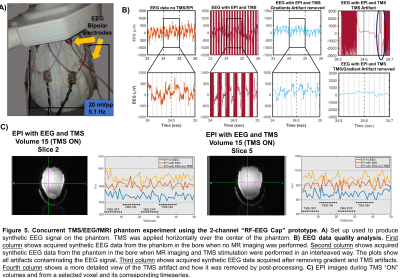
Additionally, to assess the feasibility of using the proposed technology to acquire high quality EEG data and functional imaging, we conducted a concurrent TMS/EEG/fMRI experiment using a phantom. To produce synthetic EEG data, the phantom’s head was covered with a cloth immersed in a 9% NaCl solution with the 2-channel “RF-EEG Cap” placed above it. The cloth was connected to a signal generator outside the scanner delivering a 5.1Hz square signal at 20mVpp (see Fig.5A). TMS pulses were delivered as indicated by the dots in the TMS “ON” block shown in the timeseries of Fig.5C, at 50% maximum stimulation output. Functional imaging (SL=3mm, TR=1000ms, TE=37ms, FA,90, 6slices, 2.4mmx2.4mm in-plane) was interleaved with the TMS pulses that were delivered just before the acquisition of the 5th slice (650ms after a volume starts). EEG data was recorded using the BrainAmp ExG MR 16 (Brain Products GmbH, Germany) and processed using EEGLab software package12,13.
Results
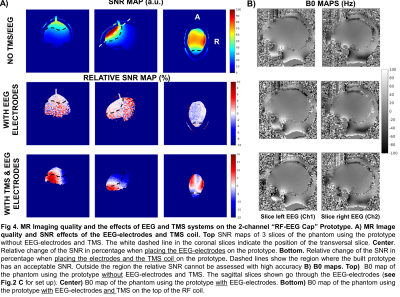
The Qu/Ql of the RF coil elements were 2.24 and 2.19. Bench test results for each condition are shown in Fig.3A and Fig.3B. A summary of the main effects on the S-parameters when adding the different modalities is shown in Fig.3C.SNR maps of the 2-channel “RF-EEG Cap” as stand-alone, without EEG-electrodes and TMS are shown in Fig.4A-Top. The relative SNR is shown for the case when the EEG-electrodes or the TMS (placed over the left hemisphere of the phantom) are included. When the EEG-electrodes are placed, no significant SNR change was observed over the region with sufficient SNR. In contrast, when the TMS coil was placed over the phantom, the well-known characterized B+1 effect of TMS14 can be observed. No additional SNR changes are expected due to the presented technology. Sagittal B0 maps directly over the EEG-electrodes are shown in Fig.4B. No significant effects on the phantom brain due to the EEG-electrodes are visible.
The results of the quality analysis of the acquired EEG and fMRI data are presented in Fig.5B and Fig.5C respectively. The TMS pulses and EEG acquisition did not affect the fMRI image quality as shown in the timeseries.
Discussion
From the findings presented above we conclude that the flexible coaxial RF technology is a feasible choice to build the proposed “RF-EEG Cap”. The shifting effect on the resonance when placing the TMS over them was reported to be less than 0.4MHz compared to 4-5MHz when using standard copper wire coils15. The minimal effects observed on the SNR, B0 maps, EEG quality signal and fMRI volumes and timeseries justify the further development of the “RF-EEG Cap” to enable high quality data acquisition for concurrent TMS/EEG/fMRI experiments.Acknowledgements
This work was funded by NIH R00EB015445, R01MH111829, NIH R00EB021349 and the Rappaport Foundation.References
(1) Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D. & Pascual-leone, A. Efficacy of Transcranial Magnetic Stimulation Targets for Depression Is Related to Intrinsic Functional Connectivity with the Subgenual Cingulate. BPS 72, 595–603 (2012).
(2) Opitz, A., Fox, M. D., Craddock, R. C., Colcombe, S. & Milham, M. P. An integrated framework for targeting functional networks via transcranial magnetic stimulation. Neuroimage 127, 86–96 (2016).
(3) Price, G. W., Lee, J. W. Y., Garvey, C. A. L. & Gibson, N. The use of background EEG activity to determine stimulus timing as a means of improving rTMS efficacy in the treatment of depression: A controlled comparison with standard techniques. Brain Stimul. 3, 140–152 (2010).
(4) Peters, J. C. et al. On the feasibility of concurrent human TMS-EEG-fMRI measurements. J. Neurophysiol. 109, 1214–1227 (2013).
(5) Peters, J. C., Reithler, J., Sack, A. T., Graaf, T. A. De & Schuhmann, T. Concurrent human TMS-EEG-fMRI enables monitoring of oscillatory brain state-dependent gating of cortico-subcortical network activity. Commun. Biol. 3, 1–11 (2020).
(6) Pruessmann, K. P., Weiger, M., Scheidegger, M. B. & Boesiger, P. SENSE: sensitivity encoding for fast MRI. Magn. Reson. Med. 42, 952–62 (1999).
(7) Griswold, M. A. et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 47, 1202–10 (2002).
(8) Auerbach, E. J., Xu, J., Yacoub, E., Moeller, S. & Uğurbil, K. Multiband accelerated spin-echo echo planar imaging with reduced peak RF power using time-shifted RF pulses. Magn. Reson. Med. 69, 1261–7 (2013).
(9) Zhang, B., Sodickson, D. K. & Cloos, M. A. A high-impedance detector-array glove for magnetic resonance imaging of the hand. Nat. Biomed. Eng. 2, 570–577 (2018).
(10) Ruytenberg, T., Webb, A. & Zivkovic, I. Shielded ‐ coaxial ‐ cable coils as receive and transceive array elements for 7T human MRI. 1–12 (2019). doi:10.1002/mrm.27964
(11) Obermann, M. et al. Optimization and miniaturization of Rx-only coaxial coil interfacing. Proc. 28th Ann. Meet. ISMRM, Virtual,2020.
(12) A Delorme & S Makeig (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics (pdf, 0.7 MB) Journal of Neuroscience Methods 134:9-21
(13) R.K. Niazy, C.F. Beckmann, G.D. Iannetti, J.M. Brady, S.M. Smith, Removal of FMRI environment artifacts from EEG data using optimal basis sets, NeuroImage, Volume 28, Issue 3, 2005, Pages 720-737
(14) Navarro de Lara, L. I. et al. Evaluation of RF interactions between a 3T birdcage transmit coil and transcranial magnetic stimulation coils using a realistically shaped head phantom. Magn. Reson. Med. 84, 1061–1075 (2020).
(15) Navarro De Lara, L. I. et al. A novel coil array for combined TMS/fMRI experiments at 3 T. Magn. Reson. Med. 74, 1492–1501 (2015).